In December 2019, an outbreak of pneumonia of unknown etiology occurred in Wuhan, Hubei Province, China, affecting more than 60 people by the 20th of the same month. On December 31, the Wuhan Municipal Health Committee informed the World Health Organization that 27 people have been diagnosed with pneumonia of unknown cause, with 7 of them were critically ill. Severe acute respiratory syndrome-related to coronavirus 2 (SARS-CoV-2) causes a pathological process that may be asymptomatic or mild to the upper respiratory tract, while in the most severe cases, respiratory distress syndrome, multiple organ dysfunction and septic shock develop [12].
A critical illness of different etiology is usually associated with hypermetabolism and severe catabolism. One of the most common methods for assessing the severity of the catabolic syndrome is to determine the nitrogen balance [3].The concept of the so-called “nitrogen balance” is that the difference between nitrogen intake and loss reflects the increase or loss of total protein in the human body. A nitrogen balance ranging from — 4 or –5 g/day to + 4 or + 5 g/day is usually considered “nitrogen equilibrium”. From a practical point of view, the determination of the nitrogen balance has its limitations. The most popular method for as sessing nitrogen balance used in clinical practice assumes that the total nitrogen loss is equal to the urinary excretion of urea nitrogen and an additional permanent loss of another 4 g/day [4, 5]. A constant ratio of 4 g/day means that 2 g of nitrogen is accounted for by non-urea nitrogen in urine, and the remaining 2 g of 4 g — on the skin and gastrointestinal tract. However, all of these assumptions underestimate non-urea nitrogen in urine (ammonia, creatinine, uric acid, amino acids) for catabolic critically ill patients and gastrointestinal nitrogen excretion in diarrhea [6].
Recently data showing that an increase in nitrogen balance for every 1 g per day increases the possibility of a positive clinical outcome of the disease (odds ratio 1.21, p = 0.03) were published [7]. It is well known that especially elderly patients, the prevailing category of ICU patients with COVID-19, have less muscle and higher fat mass than younger patients with a similar body weight, which, of course, aggravates the consequences of the progression of hypercatabolism syndrome [8, 9]. It is clear that further research is needed on the role of nitrogen balance in relation to clinical outcomes in critically ill patients.
Objectives of the study — evaluation of the prognostic value of some indicators of the severity of catabolic syndrome in COVID-19 ICU patients.
The study was organized among patients of ICU departments of Almazov National Medical Research Centre (St. Petersburg, Russia). There was a single-center, prospective, open cohort clinical study. ICU patients with a severe COVID-19 were included.
Inclusion criteria (all of the above criteria must be met):
Exclusion criteria (one is sufficient):
Duration of observation — from the moment of inclusion into the study to transfer from the ICU. Data registration regimen: during the first 72 hours, from 4th to 7th day and from 8th to 14th day of stay in the ICU.
Intensive care treatment of patients was carried out according to the current version of the Temporary guidelines. “Prevention, diagnosis and treatment of new coronavirus infection (COVID-19)” of the Ministry of Health of the Russian Federation. Version 8.1 (01.10.2020). Transfusion of 20 % human albumin solution was provided only with a decrease in the level of serum albumin to 25 g/L or less.
As criteria for the severity of the catabolic syndrome we have chosen daily urea nitrogen excretion and serum albumin level. To determine the daily nitrogen loss, urine was collected and the level of urea in urine in mmol/L was determined. The calculation of nitrogen loss was made according to the formula: nitrogen excretion (g/day) = urine urea (mmol/day) . 0.033 (g), where 0.033 is the conversion factor of urea into nitrogen.
Nutritional support was provided in accordance with the clinical guidelines of the Federation of Anesthesiologists and Reanimatologists of the Russian Federation “Metabolic control and nutritional support in patients on prolonged mechanical ventilation” [28]. Patients on non-invasive mechanical ventilation received enteral nutrition in a sipping mode with oral enteral diets in combination with a hospital diets. In patients on invasive mechanical ventilation (IMV), tube enteral nutrition through the nasogastric access was performed with the Standard and Diabetes enteral diets.
The value of the indicator variation in the study was expressed using the confidence interval (CI). The size of the analyzed population is presented as n; achieved level of significance — p. Comparative analysis of nonparametric quantitative data was carried out using the Mann–Whitney test. To determine the predictive value of the diagnostic test, we plotted using the curves of operating characteristics (ROC, Receiver Operating Characteristics) with the subsequent determination of the sensitivity and specificity at the separation point.
55 patients with severe COVID-19 were included in the study. The average age was 64.4 (21; 79) years, the average body weight was 87.7 (59.7; 112.13) kilograms. Men accounted for 56.36 % (n = 31), women — 43.64 % (n = 24). Subsequently, two subgroups of patients were formed — with a positive (n = 29) and negative (n = 26) clinical outcome.
In order to assess the possibility of predicting the development of negative clinical outcome in patients with severe COVID-19 we performed ROC analysis of the two most important indicators characterizing the severity of hypercatabolic syndrome — serum albumin and daily urinary nitrogen excretion during the first 14 days of treatment in the ICU.
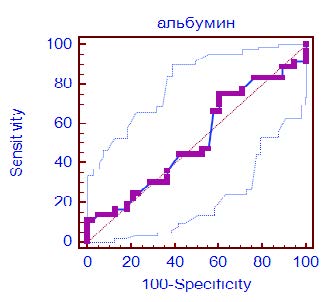
The area under the ROC curve (Fig. 1) for serum albumin was 0.516 (0.397–0.634) (CI 0.397–0.634, p = 0.81) with a sensitivity of 75 % and a specificity of 39.47 % (table 1), which indicates a low ability of the test to predict the development of negative clinical outcome. Cut-off point was determined as 29.7 g/L.
Fig. 1. ROC curve of serum albumin level effect on the clinical outcome in ICU patients with COVID-19

Analysis of the dynamics of serum albumin in survivors and non-survivors did not reveal any significant differences at all stages of the study. It is also important that in both compared subgroups serum albumin decreased moderately and fluctuated in the range of 29–32 g/L.
The area under the ROC curve (Fig. 2) for the daily nitrogen excretion was 0.624 (CI 0.49–0.76, p = 0.09) with a sensitivity of 75.9 % and a specificity of 58.1 % (table 1), which indicates a moderate ability of the test to predict the development of negative clinical outcome. The cutoff point was determined as 20 g of nitrogen per day, which indicates an increased risk of developing of negative outcome with a total daily protein loss of more than 130–135 g, taking into account extrarenal nitrogen losses.
Fig. 2. ROC curve of daily urea nitrogen excretion effect on the clinical outcome in ICU patients with COVID-19
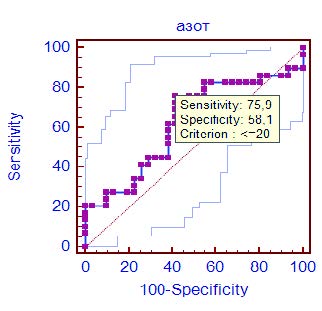
Table 1. Results of ROC analysis of daily urinary nitrogen excretion and serum albumin in patients with severe COVID-19 during 14 days of ICU stay
|
Indicator |
Area under curve |
Cut-off point |
Sensitivity (CI), % |
Specificity (CI), % |
р |
|---|---|---|---|---|---|
|
Daily urea nitrogen excretion |
0.624 (0.49–0.76) |
≤ 20 |
75.9 (56.5–89.7) |
58.1 (39.1–75.5) |
0.09 |
|
Serum albumin |
0.516 (0.397–0.634) |
≥ 29.7 |
75 (57.8–87.9) |
39.47 (24.0–56.6) |
0.81 |
It should be noted that it was found out that in survivors there was a significant increase in daily urinary nitrogen excretion from 4 to 7 days (p = 0.022) and from 8 to 14 days (p = 0.011) in comparison with a group of patients with negative clinical outcome (Fig. 3).
Fig. 3. Dynamics of daily nitrogen excretion in groups with positive and negative clinical outcome during 14 days of stay in ICU
*p < 0.05; **p < 0.01

Systemic infection, polytrauma, major surgery are accompanied by varying degrees of hypercatabolism. The severe COVID-19 is a specific type of the systemic inflammatory response, in which the manifestation of organ dysfunction, as a rule, begins with damage of the lungs. Acute lung injury is the earliest and most frequent complication in prolonged catabolic states [10]. Tissue damage caused by the SARS-CoV-2 virus and hyper immune reaction at the alveolar level is characterized by pathological changes in the form of infiltration, hyperplasia and early fibrosis. The data on the features of the catabolic syndrome in COVID-19 are still scarce, contradictory and not systematized [11].
In critical care medicine serum albumin has long been considered as a predictor of negative clinical outcome, which has been confirmed by a number of publications. In 83 critically ill patients, a decrease in plasma albumin concentration was accompanied by an increase in pulmonary vascular permeability, regardless of the underlying disease and fluid status. Blood albumin levels have shown high sensitivity and negative predictive value for predicting increased pulmonary vascular permeability and the risk of respiratory distress syndrome developing [12].
In a meta-analysis of 90 cohort studies involving more than 290,000 patients evaluating hypoalbuminemia as a predictor of negative clinical outcome using multivariate analysis, and in nine prospective controlled studies on the correction of hypoalbuminemia, in which 535 patients participated, the relationship between hypoalbuminemia and poor outcome was evident. Regardless of nutritional status and severity of the inflammatory process [13]. More recent studies including nearly 6,000 adult patients have also shown that hypoalbuminemia on admission is an independent marker of 30 day all-cause mortality [14].
Based on the results of our study, we conclude that there is a low predictive value of serum albumin in severe COVID-19. However, in March 2020, Chinese researchers published data demonstrating significantly lower blood albumin levels in non-survivedCOVID-19 patients. It should be noted that the average levels of blood albumin in our study and in the trial of Chinese colleagues were very similar (29.1–35.8 g/L) [15]. It is also important that the results of a clinical study published in 2019 assessing the prognostic levels of hypoalbuminemia demonstrated that serum albumin 24.5 g/L was defined as the optimal threshold value for predicting short-term and long-term mortality in ICU patients with septic shock [29].
In contrast, 24-hour urinary nitrogen excretion showed moderate predictive value as demonstrated by ROC analysis. Perhaps a more important finding should be considered was the revealed trend of a significant increase, rather than a decrease, as one would expect, in urinary nitrogen losses in the group of survivors. This kind of result could be substantiated by studies that explain in detail the basis of the development of muscle catabolism processes for tissue recovery after polytrauma [16, 17]. Also, in a recently published Russian study devoted to the analysis of protein metabolism disorders in thoracic and abdominal trauma, the authors described an upward trend in daily nitrogen losses with maximum values of urinary nitrogen excretion on days 7–10 of intensive care [18]. The explanation of this phenomenon could be based on several hypotheses. Firstly, high degree of catabolism is typical for the progression of the inflammatory response and maybe caused by the activation of proliferative processes that develop during severe COVID-19 [19].Secondly, the increase in catabolism in the group of survivors can be explained by the well-known concept of activation of “autophagy” as a classical mechanism of sanogenesis in critical illness. The activation of autophagy in critical illness has been previously interpreted as a negative reaction that promotes hypercatabolism and wasting. However, the hypothesis that autophagy may be protective for skeletal muscles and other organs during severe illness was initially voiced and obtained in studies performed on experimental animals (mice with autophagy selective for various tissues) [20, 21].
In fact, it has not previously been studied whether autophagy is activated enough to cope with severe cellular damage caused by critical illness. However, a clinical study that took into account the effects of insufficient autophagy confirmed similar adverse changes in skeletal muscle and liver in critically ill patients. They consisted in the accumulation of aggregates of ubiquitin and other substrates of autophagy, such as deformed mitochondria and aberrant concentric membrane structures) [22, 23].
Fasting is the most serious physiological activator of autophagy, while nutrition and insulin inhibit this mechanism [24]. Muscle biopsy performed in ICU patients showed suppression of autophagy activation with early versus late onset of parenteral nutrition, but in the absence of any effect on markers of muscle atrophy development [25]. Adequate activation of autophagy appears to have been critical to provide protection against mitochondrial dysfunction, liver damage, and renal failure in critically ill models in experimental animals [26, 27].
Some important limitations of our study should also be noted. Of course, the ICU patient is influenced by a number of factors that can reduce or increase the metabolic rate, namely: sedation and analgesia, myoplegia, the use of glucocorticoids, mechanical ventilation, infectious complications, the volume and quality of nutritional support. Since it is often extremely difficult to isolate the influence of any factor in practical setting, we proceeded from the message that these factors had an equal effect on patients in the group of survivors and non-survivors. We found out the phenomenon of increased urinary nitrogen loss in the group of survivors from 4 to 14 days. However, in the group of non-survivors, there was no decrease in nitrogen losses, nitrogen losses were initially moderately increased in comparison with normal values and did not change during the observation period. The observation period of only 14 days from the moment of admission to the ICU does not allow us to conclude that the lower the nitrogen loss in patients with COVID-19, the higher the mortality. Perhaps, if the study had been extended until the patients were discharged from the hospital, this formulation would have been more correct.
Serum albumin level during systemic inflammatory response could not be considered as a predictor of clinical outcome in severe COVID-19. In contrast, daily urinary nitrogen excretion is a more accurate prognostic marker of negative clinical outcome of this disease. The main feature of the catabolic syndrome in patients with positive outcome of COVID-19 is a progressive increase in the rate of urinary nitrogen excretion from days 4 to 14 of intensive care. However, the obtained results should not be taken straightforwardly to determine the strategy and tactics of nutritional support, in particular, aggressive enteral or parenteral loading with nitrogen sources. The revealed patterns suggest that dynamic metabolic monitoring based on methods such as indirect calorimetry, assessment of nitrogen balance, key markers of the somatic and visceral protein pool is an important approach in the practical implementation of a personalized approach to the choice of strategy and tactics of nutritional therapy. The features of the catabolic syndrome in COVID-19 certainly require further comprehensive description and analysis.
Conflict of interest. The authors declare no conflict of interest.
Contribution of authors. Leyderman I.N. — development of the concept of the article, obtaining and analyzing study data, writing and editing the text, verification and approval of the text of the article, justifying the scientific significance; Lesteva N.A. — contributions to the acquisition, analysis and interpretation of data for the work; drafting the work for important intellectual content; final approval of the version to be published; Kasherininov I.U. — contributions to the acquisition, analysis and interpretation of data for the work; drafting the work for important intellectual content; final approval of the version to be published; Kuzmin A.S. — contributions to the acquisition, analysis and interpretation of data for the work; drafting the work for important intellectual content; final approval of the version to be published; Akhimov P.S. — contributions to the acquisition, analysis and interpretation of data for the work; drafting the work for important intellectual content; final approval of the version to be published; Kanshaov N.Z. — contributions to the acquisition, analysis and interpretation of data for the work; drafting the work for important intellectual content; final approval of the version to be published; Barinova S.A. — contributions to the acquisition, analysis and interpretation of data for the work; drafting the work for important intellectual content; final approval of the version to be published; Mazurok V.A. — contributions to the design of the work, the acquisition, analysis and interpretation of data for the work; drafting the work for important intellectual content; final approval of the version to be published.