
Increased pulmonary artery pressure (PAP) is considered a factor associated with adverse outcomes of cardiac surgery in adults [1, 2] and children [3].
Increased mean pulmonary artery pressure (MPAP) ≥ 25 mm Hg, in accordance with the clinical guidelines of the European Society of Cardiology is interpreted as pulmonary hypertension (PH) [4]. Current guidelines indicate the presence of five clinical groups of PH:
I — pulmonary arterial hypertension (PAH);
II — pulmonary hypertension due to diseases of the left heart;
III — pulmonary hypertension associated with lung disease and/or hypoxemia;
IV — chronic thromboembolic pulmonary hypertension;
V — pulmonary hypertension with unclear and/or mixed mechanisms [4].
In adult cardiac surgery patients, an increase in pressure in the pulmonary circulation is caused by the presence of PH groups I, II, and IV. In pediatric cardiac surgery, in most cases PH is represented by group I conditions. This variant of PH is precapillary, since an increase in PAP is caused by hyperdynamia and/or an increase in arterial resistance in the pulmonary capillary bed [4–6]. In accordance with the modern classification, such children belong to group 1.4.4 of pulmonary arterial hypertension. This group includes patients with operable and inoperable congenital heart defects (CHD) [4, 5]. Transient PH after CHD correction occurs in 21.9 cases per million population and is one of the most common forms of PAH in children [5].
Precapillary pulmonary hypertension after cardiac surgery in children is dangerous due to the risk of crisis and the development of acute right ventricular failure [7]. Pulmonary vasodilators are of key importance in the intensive care of precapillary PH. The use of these drugs in adults and children in the postoperative period of cardiac surgery is accompanied by a significant improvement in hemodynamics and high clinical efficacy, which has been proven in many studies [7–11]. Pulmonary vasodilators used for intensive therapy of acute precapillary PH crisis and right ventricular failure must have specific requirements: a minimal effect on the systemic circulation, absence of negative inotropic effect, and should be well controlled [12]. These requirements are more consistent with inhaled nitric oxide, so this agent is the drug of choice for intensive therapy in case of precapillary PH crisis, including in the postoperative period of cardiac surgery in children [7, 11, 13–15].
From the alveolar space, nitric oxide (NO) molecules enter the precapillary and capillary. NO increases the activity of guanylate cyclase, thereby increasing the content of cyclic guanosine monophosphate (cGMP) in smooth muscle cells of blood vessels. The result is a decrease in the sensitivity of contractile proteins to Ca+2, as well as a decrease in the Ca+2 intake into the cell and a decrease in it’s release from the sarcoplasmic reticulum [16, 17]. Selective pulmonary vasodilation is due to the fact that NO has a high affinity for hemoglobin with the formation of methemoglobin. Inhaled NO does not enter the systemic circulation, therefore its pronounced vasodilatory effect is limited to the lungs [18, 19].
Today, NO is delivered to hospitals in cylinders at a concentration of 0.1 % (1000 parts per million (ppm)), at a pressure of 150 atm. In addition to the gas itself, NO supply technology requires a dosing device, the logistics of the purchase, storage and cylinders exchange. These features significantly limit the widespread use of NO inhalation therapy.
At the Russian Federal Nuclear Center — All-Russian Research Institute of Experimental Physics, an apparatus capable of synthesizing NO by the electric discharge method was created [20]. Based on the permission of the Federal Service for Surveillance in Healthcare (Roszdravnadzor), clinical trials of the medical device “Apparatus for the therapy with nitric oxide AIT-NO-01” in adult patients and children were carried out at the “Almazov National Medical Research Center” (St. Petersburg, Russia).
Aim of the study was to study the clinical efficacy and safety of using the method of synthesis of NO from atmospheric air in the postoperative period of cardiac surgery in children with precapillary PH.
Research hypothesis: NO inhalation therapy after cardiac surgery in children using the AIT-NO-01 device has the same effectiveness and safety compared with the method of NO supply from cylinders.
A single-center cohort study with retrospective control was performed in the “Almazov National Medical Research Center” (St. Petersburg, Russia), from 01.07.2019 to 12.05.2020. The protocol of this study was approved in accordance with the authorization to conduct clinical trials No. 755/2018 dated September 27, 2018 (Conclusion No. 655-1112/1-18 dated September 17, 2018 on the possibility of conducting clinical trials) of the Federal Surveillance Service in healthcare (Roszdravnadzor). In addition, the Conclusion of the Ethics Council of the Ministry of Health of the Russian Federation on the ethical justification of clinical trials of a medical device with human participation No. 17 dated January 14, 2019 was received. The research work was approved by the Local Ethics Committee of the “National Medical Research Center named after V.A. Almazov” at the meeting on 13.05.2019 (extract No. 050519 from the minutes of the meeting 06–19). The results of applying the method of NO synthesis from atmospheric air using AIT-NO-01 (clinical study group) were compared with the results obtained in the retrospective control group using a device for dispensing NO from cylinders. Patients in the retrospective control group underwent cardiac surgery in the period from January 2018 to December 2019.
Figure 1. AIT-NO-01 device for nitric oxide therapy

The following criteria for inclusion in the study were determined: signed informed consent of the patientʼs legal representative, underwent heart surgery, mechanical ventilation through an endotracheal/tracheostomy tube, systolic pulmonary artery pressure (PAPsys) ≥ 35 mm Hg according to echocardiography. Exclusion criteria: methemoglobinemia, severe left ventricular failure (NYHA functional class III and IV), intracranial hemorrhage, hemorrhagic diathesis.
In total, the study included 90 patients (28 newborns and 62 children older than 28 days), there were 40 boys and 50 girls, the median age was 0.25 (0.28; 1.17) years. 45 patients were included in the main group (using the AITNO-01 device), 45 — in the retrospective control group (using the method of NO supply from a cylinder). All patients underwent cardiac surgery. Surgery of the ventricular septal defect (VSD) was performed in 31 cases, correction of the transposition of the great vessels was performed in 21 children, plastic surgery of the atrial septal defect (ASD) — in 13 cases, tetralogy of Fallot was corrected in 4 children, the complete atrioventricular canal was corrected in 5 cases, other operations — in 16 cases. The course of the postoperative period in all newborns and children was complicated by the development of precapillary pulmonary hypertension.
The standard patient’s preparation for elective surgery included refusal to eat at least 3 hours before surgery. During surgery, all patients underwent general combined anesthesia according to a protocol adopted at the clinic Almazov National Medical Research Center. Induction into anesthesia was carried out by inhalation of one minimum alveolar concentration (MAC) of sevoflurane, fentanyl, administered intravenously at a dose of 5 μg/kg. Anesthesia was maintained by inhalation of sevoflurane (0.75–1 MAC) and intravenous infusion of fentanyl at a rate of 10 μg/kg/hour (at least 50 μg/kg before cardiopulmonary bypass and at least 100 μg/kg for the entire operation).
Standard intraoperative monitoring included the following items: invasive measurement of blood pressure (BP), placement of a central venous catheter with control of central venous pressure (CVP), determination of carbon dioxide in the end-exhaled mixture (EtCO2) and saturation of arterial blood hemoglobin with oxygen (SaO2), performed electrocardiography (ECG), rectal and nasopharyngeal thermometry, urine output.
Intraoperative mechanical controlled ventilation (MCV) was carried out with the following parameters: fraction of oxygen in the inhaled mixture (FiO2) — necessary to maintain optimal SaO2, control by volume, tidal volume (TV) — 6–8 ml/kg, respiratory rate (RR) — sufficient to maintain normocapnia.
During surgery, extracorporeal circulation was performed with a Maquet jostra HL30 apparatus (Germany) using Medtronic Affinity Pixie disposable membrane oxygenators. Connection was performed according to the standard scheme by cannulating the ascending aorta, superior vena cava and inferior vena cava. During perfusion, the following parameters were maintained: mean perfusion pressure — 30–50 mm Hg, perfusion flow — 3 l/min/m2. At the same time, pO2 in arterial blood was kept above 250 mm Hg, and pCO2 with temperature correction 30–35 mm Hg. Temperature range: moderate hypothermia. Control of systemic heparinization (calculated heparin dose 300 U/kg) was performed by measuring the activated clotting time, which was maintained above 400 sec. After removal of the shunt cannulas, the residual effect of heparin was neutralized with a solution of protamine sulfate at the rate of 1 mg per 100 U of heparin, under the control of the activated clotting time.
Myocardial protection during main stage of surgery provided by crystalloid cardioplegia with “Custodiol” solution (Dr.F. Kohler Chemie, GmbH, Germany) once.
After cardiac surgery, the patients were transferred to the ICU, where monitoring and the pharmacological support continued. Postoperative mechanical ventilation was performed with “Babylog VN 500” devices (Drager, Germany) in normoventilation mode: EtCO2 was maintained at the range of 30–35 mm Hg. TV up to 6–8 ml/kg, SpO2 at the optimal range for CHD, used a positive endexpiratory pressure (PEEP) from 5 to 7 cm H2O. Initially, the synchronized intermittent mandatory ventilation (SIMV) mode was used, followed by the transition to such modes of assist ventilation as biphasic positive airway pressure (BiPAP), as well as BiPAP in combination with pressure support (PS). After restoration of consciousness and muscle tone, continuous positive airway pressure (CPAP) was used before extubation. After extubation, insufflation was performed with a humidified oxygen air mixture through nasal catheters with a flow of 3–7 L/min.
All patients included in the study had precapillary PH in the postoperative period (calculated PAPsys ≥ 35 mm Hg according to echocardiography), which served as an indication for the appointment of NO inhalation. The supply of NO to the breathing circuit of the ventilator with a target concentration in the inhaled mixture of 20 ppm was continued until the patients were transferred to spontaneous breathing. The supply line of the mixture containing NO was connected to the inspiratory line at a distance of at least 30 cm from the endotracheal tube. In patients of the main group, NO was synthesized from atmospheric air by the AIT-NO-01 device; in the retrospective control group, gas was supplied to the patients from cylinders using the NOXBOX Mobile device (Bedfont, Great Britain). The duration of NO inhalation using the AIT-NO-01 device varied from 1 to 171.3 hours. In the retrospective control group, the duration of use of the NOXBOX Mobile device varied from 1 to 162 hours.
The complex of hemodynamic parameters of the systemic and pulmonary circulation was assessed for evaluation of NO inhalation therapy effectiveness, as well as indicators of the postoperative period. PAPsys dynamics was assessed by echocardiography and blood oxygen saturation from the right atrium (SvO2). The time of respiratory support and the length of ICU stay were selected for evaluation of postoperative period.
For assessment of the safety of NO delivery methods, we registered the development of nitric oxide therapy side effects — an increase in the concentration of nitrogen dioxide (NO2) in the inspiratory line by more than 2 ppm over the entire period of NO inhalation. In addition, the content of methemoglobin in arterial blood was assessed.
Statistical analysis was performed using the Statistica 7.0 (Statsoft Inc., USA). The normal distribution of the obtained data was checked using the Shapiro — Wilk test. Given the nature of the distribution, which is different from normal, intergroup comparisons of quantitative indicators were carried out using the Mann-Whitney method for unrelated samples and using the Wilcoxon test for related samples. Comparison of qualitative data was performed using Fisherʼs exact test. Data are presented as arithmetic mean ± standard deviation (M ± SD) with normal distribution and as median and 25th; 75th percentile (Me (Q1; Q3)) with non-normal distribution. The critical level of significance of the differences was taken as p = 0.05.
Anthropometric indices and data on pressure in the pulmonary circulation before the appointment of NO therapy in patients of the study and retrospective control groups are presented in Table 1. There were not statistical differences in the anthropometric characteristics of newborns and children, as well as in the manifestations of precapillary pulmonary hypertension before the initiation of NO therapy, which made it possible to continue the comparison of study and retrospective control groups.
Table 1. Data on newborns and children included in the comparison groups, Me (Q1; Q3)
| Parameter | Study group (n = 45) |
Retrospective control group (n = 45) |
|
|---|---|---|---|
| Age (years) | 0.42 (0.08; 1.4) | 0.25 (0.08; 0.42) | |
| Sex | male | 15 (33.3) | 25 (55.6) |
| female | 30 (66.7) | 20 (44.4) | |
| Newborns (n/%) | 13 (28.9) | 15 (33.3) | |
| Weight (kg) | 5,7 (3,5; 9,95) | 4.9 (3.7; 6.2) | |
| Height (cm) | 63 (54; 79) | 60 (54; 68) | |
| Body surface area (m2) | 0.31 (0.22; 0.47) | 0.29 (0.24; 0.34) | |
| PAPsys (mm Hg) | 45 (42; 45) | 45 (42; 50) | |
| No statistically significant differences between groups were found when comparing all parameters. | |||
Data on changes in hemodynamic parameters in response to the initiation of NO therapy using the AITNO-01 device and in the retrospective control group are presented in Table 2.
Table 2. Hemodynamic parameters in the comparison groups at the initial stage and after one hour of therapy with nitric oxide, Me (Q1; Q3)
| Parameter | Study group (n = 45) |
Retrospective control group (n = 45) |
|---|---|---|
| Initial PAPsys (mm Hg) | 45 (42; 45) | 45 (42; 50) |
| PAPsys (mm Hg) after 1 hour of NO therapy | 30 (24; 35) | 33 (25; 36) |
| The number of patients with decreased PAPsys by 10 % or more, n (%) | 40 (88.9 %) | 38 (84.4 %) |
| Initial BPmean (mm Hg) | 67 (57; 74) | 66 (58; 77) |
| BPmean (mm Hg) after 1 hour of NO therapy | 63 (53; 68) | 65 (56; 70) |
| Initial CVP (mm Hg) | 8 (7; 10) | 9 (7; 11) |
| CVP (mm Hg) after 1 hour of NO therapy | 9 (7; 10) | 9 (6; 10) |
| Initial HR (beat/min) | 144 (132; 158) | 148 (135; 155) |
| HR (beat/min) after 1 hour of NO therapy | 150 (136; 155) | 149 (137; 159) |
| Initial ScvO2, % | 77 (69; 86) | 77 (70; 85) |
| ScvO2 after after 1 hour of NO therapy, % | 76 (70; 83) | 78 (69; 87) |
As follows from the data given in Table 2, after one hour of NO inhalation using the AIT-NO-01 device (study group), a significant decrease in PAPsys was noted (by 33.3 %, p < 0.001). In the retrospective control group, after one hour of NO inhalation, a statistically significant decrease in PAPsys was also noted (by 26.7 %, p < 0.001). PAPsys decreased by 10 % or more in 40 (88.9 %) of patients in the study group (AIT-NO-01 device) and in 38 (84.4 %) of patients in the group of retrospective control (NOXBOX Mobile device), the difference between the groups is not significant (p = 0.4). Thus, inhalation therapy of NO using the method of NO synthesis from atmospheric air was no less effective in reducing pressure in the pulmonary circulation than with the method of supplying gas from cylinders (Fig. 2).
Figure 2. Pulmonary artery systolic pressure was significantly reduced when using both methods of delivery of nitric oxide
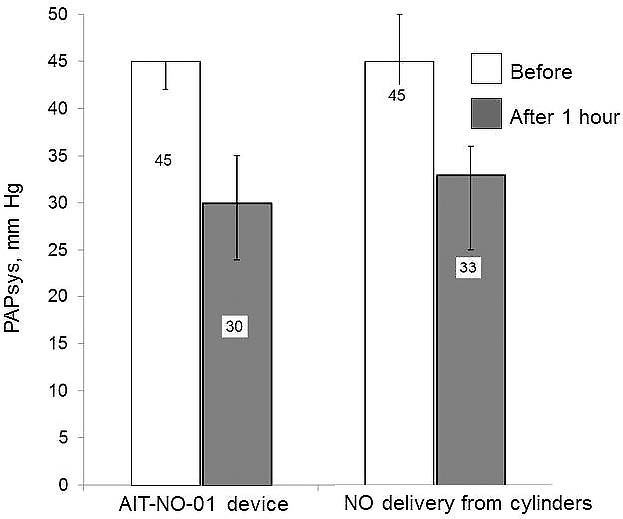
We believe that in patients with precapillary pulmonary hypertension, the detected decrease in pulmonary artery pressure against the background of using both methods of NO delivery had a positive effect on the state of the right ventricle. The study performed did not reveal any significant effects of NO therapy on blood pressure and heart rate. Changes in these indicators were not found both when using AIT-NO-01, and when applying the method of supplying NO from cylinders (Table 2). Likewise, there were no significant changes in the ScvO2 one hour of NO therapy. The use of AIT-NO-01 and the use of the cylinders method were accompanied by the maintenance of adequate oxygen delivery to tissues and organs.
When NO inhalation was supply in the postoperative period using the AIT-NO-01, the median time of mechanical ventilation was 12 (2; 28) h. In the retrospective control group, when using the cylinders method, the median of this indicator was 14 (12.2; 70.5) h, the difference between the groups is statistically significant (p = 0.01). The shorter time of mechanical ventilation when using the method of NO synthesis from atmospheric air, in our opinion, was due to absence of pauses in NO inhalation therapy. There were 13 pauses in NO inhalation therapy when using the method of gas delivery from cylinders. In 4 cases pauses were due to exceeding NO2 content more than 2 ppm. In another 9 cases, temporary cessation of inhalation in the retrospective control group were associated with a change of cylinders. The length of stay of patients in the ICU did not differ between the groups and was 96 (21.7; 168) h when using the AIT-NO-01 device and 112 (22.4; 196) h when using the cylinders method of NO supply.
In the group of children who received inhalation therapy using the AIT-NO-01 device, there were 14 (31.1 %) cases of postoperative complications. The most frequent complication noted in 8 children was low cardiac output syndrome, which required the inotropic therapy. In two cases paresis of the right dome of the diaphragm caused by intraoperative damage to the phrenic nerve were found. One newborn had a complete atrioventricular block with the need for temporary pacing. The development of paroxysmal supraventricular tachycardia, which required pharmacological correction, was recorded once. One child developed intestinal paresis and cholestasis in the postoperative period. The cause of the gastrointestinal dysfunction was previously performed laparotomy and resection of intestine portion (1 month before cardiac surgery). There was one death in this group. The cause of death was multiple organ failure associated with severe sepsis caused by pneumonia. These complications were not associated with the use of the AIT-NO-01 device; the lethal outcome developed seven days after the cessation of NO therapy.
There were 15 (33.3 %) cases of postoperative complications in the retrospective control group. Seven children developed low cardiac output syndrome. Temporary cardiac pacing required in three cases. There were two cases of bleeding with required reoperation. Lungs atelectasis required tracheal re-intubation and transfer to mechanical ventilation was registered in two cases. One patient had a short, single episode of seizures. One child died in the retrospective control group. The cause of death was profuse pulmonary hemorrhage. Bleeding occurred 10 days after the cessation of NO inhalation, and death occurred on the 15th day after NO therapy.
The difference between the study groups in the postoperative complications rate was not significant (p = 0.5).
The data on methemoglobin blood content in both groups are presented in Table 3.
Table 3. Methemoglobin blood content (%) in patients during inhalation therapy with nitric oxide, Me (Q1; Q3)
| Groups of patients | Before NO inhalation | 1 hour of NO inhalation | 6 hours of NO inhalation |
12 hours of NO inhalation |
|---|---|---|---|---|
| Study group, n = 45 |
1.6 (1.4; 1.8) | 1.9 (1.6; 2.5)* | 2.6 (1.9; 2.8)* | 2 (1.4; 2.7)* |
| Retrospective control group, n = 45 |
1.1 (1; 1.5) | 2,2 (1.9; 2.6)** | 2.4 (2.1; 2.8)* | 2.5 (1.8; 2.9)* |
| * p < 0.05, when compared with the initial values; ** p < 0.01, when compared with baseline values. | ||||
We found a statistically significant increase in the methemoglobin content upon inhalation of NO in comparison with the baseline values. An increase in the methemoglobin content was characteristic both for the cases of using the AIT-NO-01 installation and for the application of the NO supply cylinders method. There were no intergroup differences in the content of methemoglobin. There were no cases of methemoglobin exceeding the threshold level of 5 % both when using the AIT-NO-01 device, and when using the cylinders method. The data obtained indicate the same safety of the method for synthesizing NO from atmospheric air and the method of NO supplying from cylinders with respect to the formation of methemoglobin.
We did not observed cases when NO2 concentration exceeded 2 ppm using AIT-NO-01 device, which is possibly due to the presence of an NO2 adsorption unit in the apparatus. We registered 4 cases of excess NO2 concentration of 2 ppm in retrospective control group, the difference between the groups was insignificant (p = 0.058). The AIT-NO-01 device is equipped with an electronic system for automatic instant stopping of the gas mixture supply when the critical concentration of NO2 in the circuit is exceeded (in our study, a level of 2 ppm was chosen). Cases of increased NO2 concentration required manual shutdown of inhalation, when using the cylinders method of NO delivery, which inevitably prolongs the patientʼs exposure to toxic NO2 concentrations.
It should be noted that in the presented study we used a relatively low NO concentration (20 ppm), which is not characterized by the formation of a significant amount of NO2. It can be assumed that the use of NO in a high dose (more than 40 ppm), especially in combination with high FiO2 (more than 50 %), will be accompanied by a high risk of reaching toxic concentrations of NO2. NO2 adsorption unit in the AIT-NO-01 device will allow more efficient control NO2 concentration versus cylinders method of NO delivery in such situations. In general, it can be argued that the application of the NO synthesis method implemented in the AIT-NO-01 device is safer that the method of NO supply from cylinders. This position is confirmed by the absence of cylinders with NO in a concentration of 1000 ppm, under a pressure of 150 atm in the immediate vicinity of the patient and the staff. Instantaneous cessation of NO synthesis when the NO2 content in the breathing circuit exceeds 2 ppm and the NO content exceeds 100 ppm, eliminates the possibility of toxic effects of these gases. The presence of an adsorber in the apparatus makes it possible to significantly reduce the formation of NO2 even high NO and oxygen concentrations used.
The above data were obtained during clinical trials of the AIT-NO-01 device, which became the basis for the issuance of a registration certificate for this medical device by the Federal Service for Surveillance in Healthcare.
Information about the study. The protocol of this study was approved in accordance with the permission to conduct clinical trials No. 755/2018 dated 27.09.2018 (Conclusion No. 655-1112/1-18 dated 17.09.2018 on the possibility of conducting clinical trials) Federal Service for Supervision of Healthcare (Roszdravnadzor). In addition, the Conclusion of the Ethics Council of the Ministry of Health of the Russian Federation on the ethical validity of clinical trials of a medical device with human participation No. 17 dated 14.01.2019 was received. The research work was approved by the local Ethics Committee of the Almazov National Medical Research Centre at the meeting on 13.05.2019 (extract No. 050519 from the minutes of the meeting 06-19).
Conflict of interest. The authors declare no conflict of interest.
Contribution of the authors. All authors according to the ICMJE criteria participated in the development of the concept of the article, obtaining and analyzing factual data, writing and editing the text of the article, checking and approving the text of the article; individual additional contribution: Bautin A.E. — conceptualization, project management; Selemir V.D. — project management; Nurgalieva A.I. — data visualization, project management; Buranov S.N., Karelin V.I., Shirshin A.S., Valueva Yu.V — providing tools, project management.