According to the recently published data of the International Diabetes Federation (IDF) the number of people with diabetes mellitus (DM) worldwide increased from 151 million in 2000 to 463 million (9.3% of the world’s population) in 2019 [1]. Meanwhile, the number of deaths worldwide resulting from DM and its complications was registered to be 4.2 million in 2019 [1]. In turn, 465,900 deaths (8.5% of all-cause mortality) in adults aged 20–79 years were attributed to DM and its complications in Europe [1]. In Europe the highest number of diabetes-related deaths (59.0%) in this year was seen in the middleincome countries, including the Russian Federation, Turkey and Ukraine [1]. Today, a huge amount of clinical evidence demonstrates the association between intra-/post-operative hyperglycaemia and the development of complications in DM patients after surgical procedures [2-7]. Notably, after cardiac surgery, increased morbidity and mortality were observed because of reduced myocardial function, wound infection, postoperative renal failure and delayed stroke [5,6]. In a recently published retrospective analysis of 44,408 patients after bariatric surgery, an increased 30-day postoperative mortality was also revealed for diabetic patients who were treated with oral hypoglycaemic agents (OR 2.58 with 95% CI: 1.44–4.62) or insulin (OR 4.96 with 95% CI: 2.74-8.97) [7]. Surprisingly, a big variation in the optimal glycaemic target ranges between hospitals in Europe, and therefore the anaesthetic management of DM patients [8]. A large multicentre trial involving mixed medical/surgical intensive care unit (ICU) patients found that intensive glucose control with a target blood glucose (BG) range of 4.5 to 6.0 mmol/L increased the absolute risk of death at 90 days by 2.6 % compare to BG target of 10.0or less mmol/L among adults in the ICU [9]. The purpose of this article is to review international publications on DM patients undergoing surgery, and discuss the current trends and problems in the management of diabetes mellitus in anaesthesia and intensive care.
Publications for analysis were selected by searching the PubMed database for the following keyword combinations: “perioperative + hyperglycemia”, “perioperative + hyperglycemia + diabetes + anesthesia”, and “perioperative + hyperglycemia + insulin”. In total, 1327 articles were selected, of which 96 were included in the review; the selection process is shown in Fig. 1. Literature sources published from 1970 to 2021 were included in the review. The main criteria for the selection of literature sources for citation in this review were experimental or clinical research studies published in journals with double independent peer review, the relevance and novelty of the published data, as well as the practical recommendations of the professional communities of anesthesiologists in Western Europe and the United States.
Fig. 1. PRISMA flowchart for selection of publications

According to the World Health Organisation (WHO) guidelines [10] the diagnosis of DM requires the presence of clinical symptoms (polyuria, polydipsia, unexplained weight loss, etc). Different tests are used to diagnose DM: random plasma glucose is more than 11.1 mmol/L or when fasting plasma glucose is more than 7.0 mmol/L on two separate occasions or a glucose concentration of more than 11.1 mmol/L two hours after oral ingestion of 75 g glucose (normal range is less than 7-8 mmol/L). Additionally, the cut-off point of 6.5% glycated haemoglobin (HbA1c) reflecting average increased plasma glucose over the previous 8-12 weeks, is also recommended for diagnosing diabetes [10]. However, a value of less than 6.5% does not exclude diabetes diagnosed using glucose tests [11].
Based on pathogenic mechanisms, DM is classified as either “type 1 or insulin-dependent” (failure in production of endogenous insulin) or “type 2 or insulin-independent” (insulin resistance, impaired insulin secretion or increased glucose production). DM “type 1 or insulin-dependent” (classified also as childhood-onset) (DM1) is characterised by deficient insulin production and requires daily administration of insulin. In turn, DM “type 2 or insulinindependent” (classified also as adult-onset) (DM2) results from the body’s ineffective application of insulin. The majority of all DM patients have DM2. DM2 is considered to be the result of excess body weight and physical inactivity and/or a genetic disposition. Clinical symptoms of DM 2 are often subtle and therefore the DM may be diagnosed after it’s complications have already developed. Additionally, the WHO classification includes other types of diabetes as hybrid types slowly evolving, immune-mediated diabetes of adults, and specific types monogenic diabetes: defects of β-cell function or insulin action, diabetes induced by: diseases of the exocrine pancreas, endocrine disorders, drug or chemical substances, infection: congenital rubella, coxsackie virus, immune-mediated reactions, genetic syndromes, and Diabetes Mellitus in pregnancy and unclassified diabetes.
Microvascular and macrovascular pathologies such as retinopathy, neuropathy and nephropathy are well known complications of long-term hyperglycaemia in DM [12]. Today, the most studied molecular mechanisms for these complications include: activation of polyol, diacylglycerol (DAG)/protein kinase C (PKC) and hexosamine pathways, advanced glycation end (AGE) products formation, and oxidative stress (formation of reactive oxygen species) [13,14].
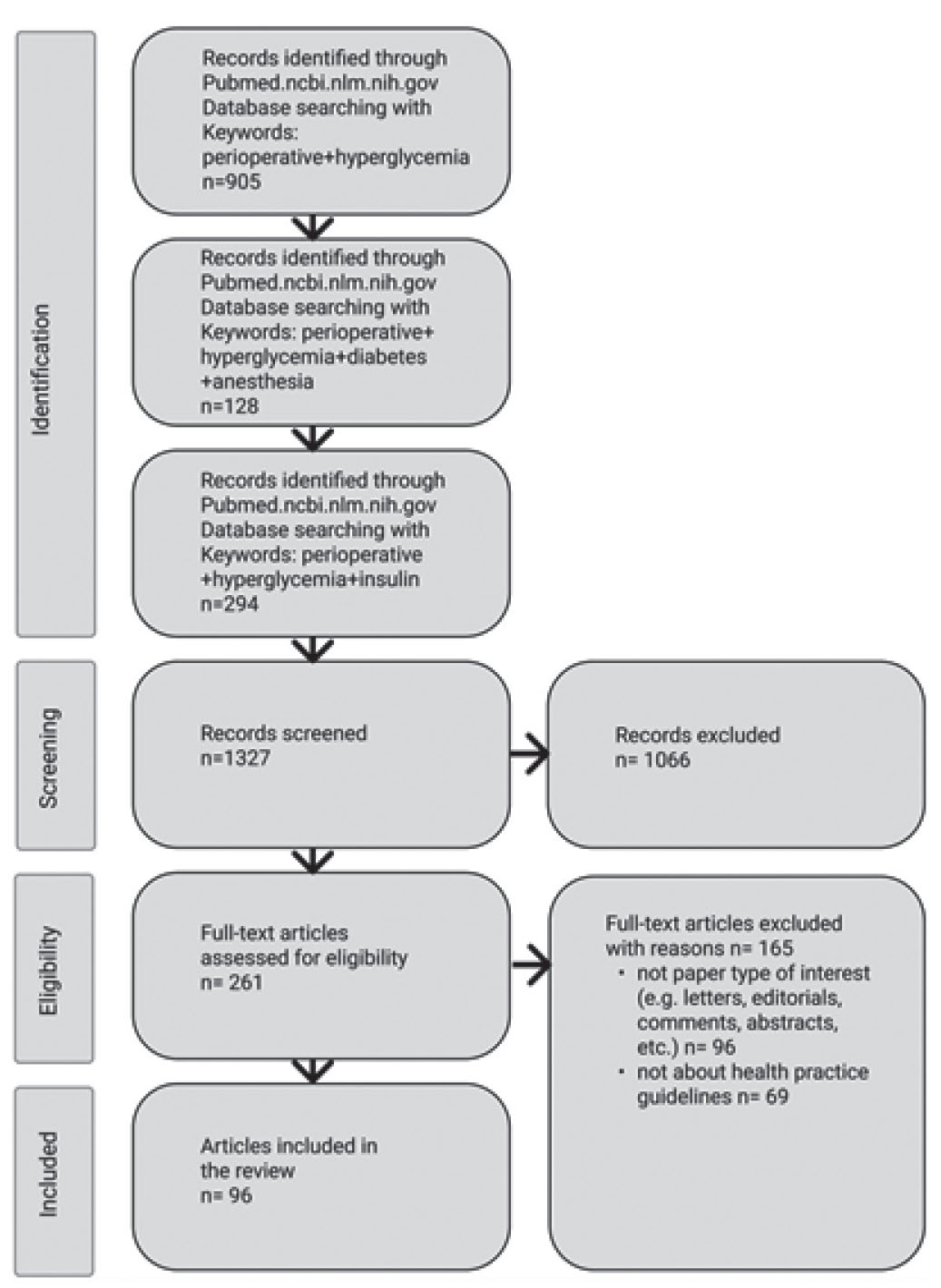
While most cells in the human body require insulin to move glucose into the cell, transport of glucose into endothelial cells of the retina, nervous and kidney tissues is insulin-independent [13-15]. Thus, glucose moves freely across the cell membranes because of differences in glucose concentration between intra- and extracellular spaces [13-15]. Excess glucose not used for energy enters into the polyol pathway, a two-step process converting glucose to sorbitol, a monosaccharide with six carbon alcohol groups (polyol) and further to fructose (Fig. 2) [13-15]. At normal concentrations of glucose, aldose reductase (AR), the enzyme for converting glucose to sorbitol has a low affinity for glucose and therefore there is no problem with intracellular accumulation of sorbitol [13-15]. However, during hyperglycemia the flux towards the polyol pathway is increased, causing accumulation of sorbitol in the cells. Sorbitol cannot cross free cell membranes, and when it accumulates it increases osmotic pressure and draws water into the cells [16]. Nicotinamide Adenine Dinucleotide Phosphate (NADPH), an essential electron donor is consumed for the converting of glucose to sorbitol, leaving less NADPH for other processes of cellular metabolism [13]. The low intracellular concentration of NADPH decreases synthesis of glutathione, nitric oxide (NO), myo-inositol, and taurine. Glutathione is an antioxidant that is capable of preventing damage of important cellular components caused by reactive oxygen species (ROS) such as free radicals, peroxides, lipid peroxides, and heavy metals [17]. Nitric oxide causes arteries and the surrounding smooth muscle to relax, resulting in vasodilation and increasing blood flow [18]. Myo-inositol is a carbocyclic sugar that is required as a secondary messenger in a number of intracellular signal transduction pathways such as cytoskeleton assembly, intracellular calcium (Ca2+) concentration control, cell membrane potential maintenance, breakdown of fats, gene expression, etc. [19,20]. Myo-inositol also participates in the intracellular signal transduction in response to a variety of hormones, neurotransmitters, and growth factors and therefore contributes to the normal function of neurones [19,20]. Finally, activation of the polyol pathway results in inhibition Na+/K+ - ATPase pumps, accumulation of intracellular Na+ , swelling of axons, reduction of nerve conduction velocity, and loss of NO-mediated vasodilatation in microcirculation.
Figure 2. Effects of long-term hyperglycaemia on polyol pathway activation.
In long-term hyperglycaemia, chronic activation of the DAG–PKC pathway results in different vascular abnormalities of the retinal, renal, neural and cardiovascular tissues [21]. In intracellular signalling, DAG is a second messenger and it is a product of the hydrolysis of the phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) by the enzyme phospholipase C (PLC). The production of DAG in the cell membrane facilitates translocation of PKC from the cytosol to the plasma membrane. PKC exists as a set of different isoenzymes: classical (α, β, γ), novel (ε, δ, θ, η) and atypical (ξ, λ) where the classical and the novel isoforms are sensitive to changes in intracellular calcium and DAG [21-26]. However, difficulties still exist in determining the individual contributions of each of the PKC isotypes to any particular process in the cell [22]. In in-vitro studies, activation of PKCα has been shown to mediate the disruption of vascular endothelial cadherin junctions [23]. Moreover, PKCα also activates myosin light chain kinase, which is involved in endothelial cell gap formation and barrier dysfunction [24-26]. Furthermore, activation of PKC leads to increased vasoconstriction and altered capillary permeability due to overproduction of endothelin-1 (ET-1). ET-1 has also been found to be a powerful pro-inflammatory peptide [27-30]. In cultured human monocytes, ET-1 stimulates release of TNF-α, IL-1β and IL-6 [29]. After being added to the pulmonary circulation of healthy rats, ET-1 caused leukocyte adhesion, platelet aggregation and histological changes indicating interstitial lung oedema [27-29]. Finally, increased DAG/PKC activation have been associated with significant changes in blood flow, basement membrane thickening, extracellular matrix expansion, increases in vascular permeability, abnormal angiogenesis, excessive apoptosis and reduction in activity of enzymes such as Na+/K+ATPase, phosphoinositide 3-kinase and mitogen-activated protein kinase.
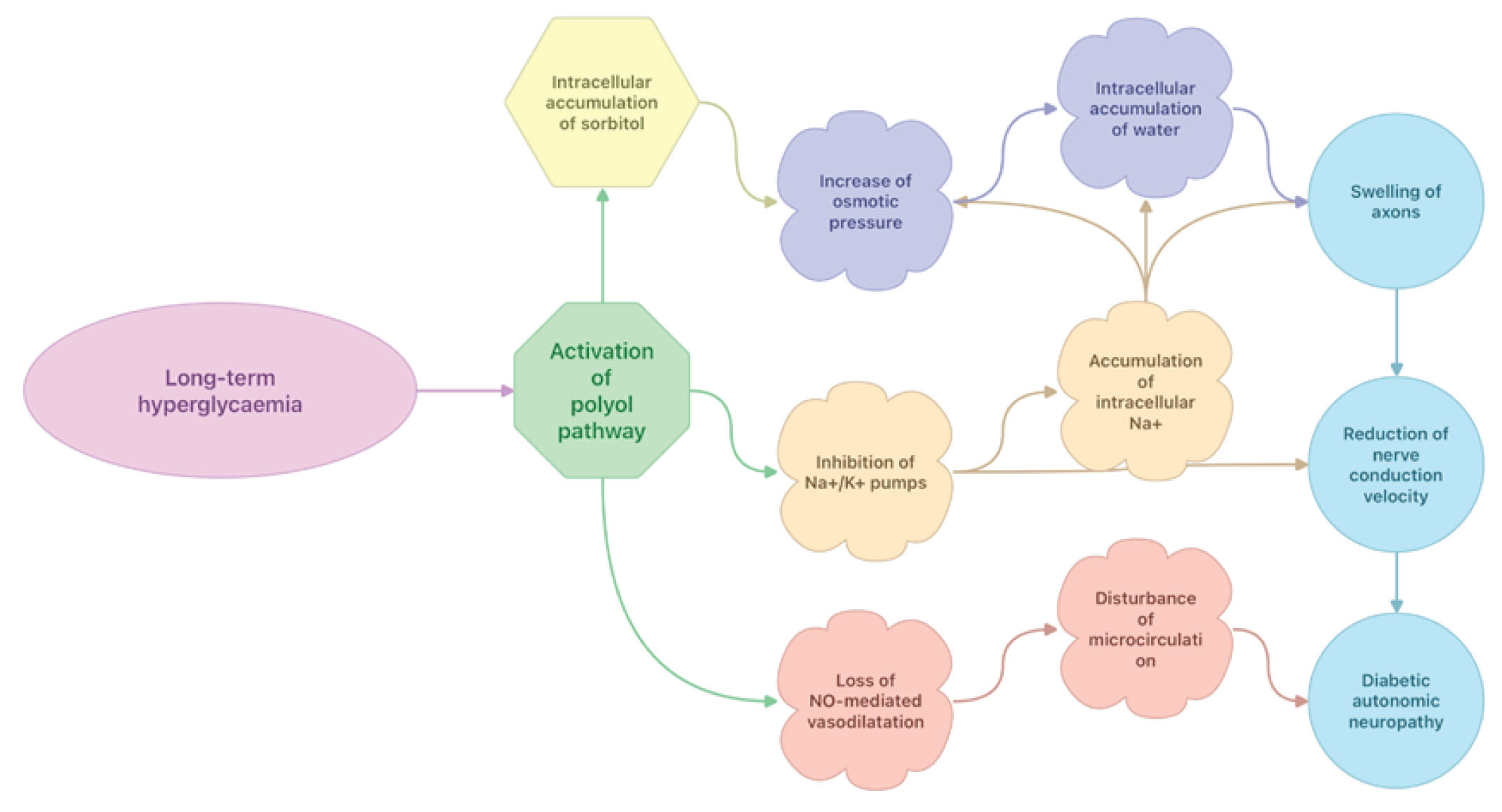
The HBP is involved in the development of insulin resistance and diabetic vascular complications [31, 32]. Most glucose taken up by the cells is metabolised through glycolysis, and at normal conditions only 2-5% of glucose enters to the HBP [33]. With long-term hyperglycaemia, some of the excess glucose is shunted into the HBP, the pathway that is responsible for the production of a key substrate for protein glycosylation, uridine-diphosphate-N-acetylglucosamine (UDP-GlcNAc) (Fig. 3) [33]. The most critical downstream utilisation of UDP-GlcNAc is the nutrient- and stress-responsive post translational modification of proteins [33]. Metabolite supply of the HBP is enhanced when glycolysis is limited as it occurs with increasing amounts of free fatty acids. Interestingly, the HBP is activated not only by increasing but also rapidly decreasing glucose concentrations or changing intracellular calcium levels [33]. The UDP-Glc-NAc plays an important role in reversible post translational protein modification and may have special impact in signal transduction [33]. The linkage of UDP-GlcNAc to proteins is called glycosylation and takes place in the Golgi apparatus. The glycosylation of proteins leads to formation of various protein alterations such as proteoglycans and glycoproteins. The HBP is highly responsive to glucose levels in blood, and its flux is significantly increased in some tissues of patients with DM, leading to increased levels of UDP-GlcNAc and, thus, elevated glycosylation of O-linkage in N-acetylglucosamine (O-GlcNAc) [33]. Sustained increases in O-GlocNAc at the cellular level alters the response of several key signalling pathways to stress. To date, many different proteins are known to be modified by O-GlcNAc, including transcription factors, kinases, phosphates, cytoskeletal proteins and nuclear hormone receptors.
Figure 3. Effects of long-term hyperglycaemia on hexosamine biosynthetic pathways.
Advanced glycation end (AGE) products formation and oxidative stress
AGE products are modifications of proteins or lipids that become glycated due to long-term hyperglycaemia [34,35]. The first step of the protein glycation process results in formation of Schiff bases and Amadori products. Further glycation of proteins and lipids cause molecular rearrangements that lead to the generation of AGE products [34]. Through activation of the glycation reaction and electron transport chain in mitochondria long-term hyperglycaemia may also induce overproduction of ROS that induces cytotoxicity, DNA damage and cell apoptosis [13,36]. AGE products accumulate in the vessel wall where they may perturb both cell structure and function [37]. The AGE products accumulation is involved in both the microvascular and macrovascular complications of diabetes [37]. Moreover, AGE products may accumulate in peripheral nerves and also contribute to development of diabetic neuropathy [37]. AGE products reduce the activity of endothelium-derived NO and therefore contribute to development of atherosclerosis. Today, it is well known that NO inhibits many of the mechanisms that contribute to atherosclerosis, such as leukocyte adhesion to the vessel wall, vascular smooth muscle growth, and platelet adhesion and aggregation [38-40]. Finally, polyol pathway overactivity and accumulation of sorbitol may also glycate nitrogens on proteins, such as collagen, and it is also the AGE products, which in turn generate ROS in a number of ways and all these changes result in the microvascular and macrovascular pathologies.
Hyperglycaemia may be revealed pre-operatively in both diabetic and non-diabetic patients. Both the excitement before surgery and the surgical operation gradually increase the secretion of stress hormones such as cortisol, glucagon, growth hormone and catecholamines that in turn cause decreased insulin secretion, and decreased peripheral utilisation of glucose [41,42]. As a consequence, gluconeogenesis and glucogenolysis increase, which subsequently results in worsening the hyperglycaemia termed as a stress-related hyperglycaemia [41, 42]. The uncontrolled stress-related hyperglycaemia instigates osmotic diuresis with further fluid and electrolyte imbalance, and increased generation of pro-inflammatory cytokines. All these changes may lead to development of diabetic ketoacidosis, immune deregulation and insulin resistance (Fig. 4).
Figure 4. Pathophysiological effects of stress-related hyperglycaemia.

In surgical patients with DM1, lengthy pre-operative fasting in combination with insulin treatment cessation and the overproduction of stress hormones will lead to enhanced lipolysis and proteolysis. The catabolic effect of these stress hormones results in increased gluconeogenesis and glucogenolysis, breakdown of fats into free fatty acid (FFA) and glycerol and proteins into amino acids. Increased levels of glycogenic precursors (glycerol and amino acids) facilitate gluconeogenesis and worsens hyperglycaemia [43]. In turn, FFA is bound to albumin and transported to the liver, where it undergoes conversation to ketone bodies. The primary ketone bodies: β-hydroxybutyrate and acetoacetic acid have a major responsibility for the development of metabolic acidosis [43]. Acetoacetic acid is metabolised further to acetone, another important ketone body. Depletion of hepatic glycogen stores stimulates further ketogenesis [43]. In surgical patients, DKA can occur very quickly and it may develop in less than 24 hours. Osmotic diuresis due to hyperglycaemia exacerbates renal potassium losses and chloride is retained in exchange for the ketoanions being excreted. The loss of ketoanions in urine is usually combined with a reduction of bicarbonate. In face of the marked ketonuria, a hyperchloremic acidosis is in progress.
The stress-related hyperglycaemia contributes to an activation of an inflammatory state characterised by an elevation of pro-inflammatory cytokines such as TNF-α, interleukin-1β, -6, and -8 [44,45]. Meanwhile, high glucose levels also suppress production of anti-inflammatory cytokines IL-2 and IL-10 [46]. The inflammatory immune response is considered to develop due to adipocyte apoptosis and macrophage infiltration and activation. Thus, the stress-related hyperglycaemia can suppress various aspects of immune function: chemotaxis, phagocytosis, generation of reactive oxygen species, and intracellular killing of bacteria [44]. All these changes result in increased vulnerability to infections and the gradual development of multi-organ system dysfunction. However, these immune deregulations may return to near-normal values with insulin therapy and hydration within 24 h.
The stress-related hyperglycaemia and further catabolic metabolism induces a development of insulin resistance. Insulin resistance is a state when the ordinary biological response to insulin is significantly reduced at any given concentration of insulin [47]. The degree of surgical trauma also contributes to this phenomenon [48-50]. Indeed, thoracic and major abdominal surgery elicit a more profound and prolonged insulin-resistant state than lowerrisk peripheral or diagnostic procedures [49]. Moreover, insulin-resistance occurs to a lesser degree in laparoscopic surgery compared to open procedures [50]. The state of insulin-resistance can promote further enhanced lipolysis, increases production of FFA, and thus creates a vicious cycle in the development of DKA. In surgical patients, an insulin resistant state may also lead to several adverse effects such as impaired muscle glycogen synthesis, reduced triglyceride uptake resulting in hypertriglyceridemia, increased hepatic glucose output, impaired NO release and function, and increased production of pro-coagulant factors. The postoperative insulin resistance may persist 2-3 weeks after uncomplicated elective abdominal surgery and therefore the state can significantly influence postoperative recovery of both diabetic and non-diabetic patients [51].
Today, the incidence of severe morbidity and mortality in association with elective anaesthesia is low. It may create a false impression that all modern anaesthetic agents effectively suppress almost all stress reactions and therefore prevent development of hyperglycaemia in the perioperative period. However, the incidences of peri- and postoperative hyperglycaemia may be revealed in 20-80% of all cases depending on the type of elective surgery, with the highest rate registered in cardiac surgery [52-54]. This is one of the reasons why opioids at high doses are still applied in cardiac surgery, because it may suppress hypothalamic and pituitary hormone secretion. Meanwhile, the stimulatory effects of cardiopulmonary bypass are so profound that peri- and post-operative hyperglycaemia still may appear in many patients [55]. Despite the effective suppression of stress hormone secretion, opioids in high doses are unsuitable for elective non-cardiac surgery because of prolonged recovery and increased need for post-operative ventilatory support [56]. Increased sympathoadrenal activity may be developed not only due to surgical trauma or stress, but also hypoxia, hypercarbia, blood loss and low arterial pressure may contribute [57]. Marked insulin resistance and therefore peri- and post-operative hyperglycaemia may develop in surgical patients during upper abdominal surgery, even when the endocrine response is minimal or absent [58]. The state of insulin resistance, which can be easily simulated by infusion of stress hormones in healthy volunteers [59], may also be caused by elevated levels of pro-inflammatory cytokines such as TNF-α [60,61] or IL-6 [62]. The elevated level of IL-6 in surgical patients is considered to be an early marker of tissue damage and this elevation is usually proportional to the degree of tissue damage [63,64]. Moreover, the endothelial and white blood cells start to synthesise IL-6 in response to increased levels of IL-1β and TNF-α [65]. Immune deregulation due to tissue damage and increased productions of the pro-inflammatory cytokines may also have a responsibility in the development of both insulin resistance and peri- and post-operative hyperglycaemia, independently of the type of anaesthesia.
Using isotope tracer technique for the investigation of intra-operative glucose metabolism kinetics (i.e., wholebody glucose production, glucose uptake and clearance), Lattermann et al. demonstrated that the increase of blood glucose by 40% from baseline observed during anaesthesia with isoflurane in patients without DM was a consequence of both impaired glucose clearance and increased glucose production [66]. In turn, supplementation with epidural analgesia to anaesthesia with isoflurane in these patients prevented effectively the hyperglycaemic response to surgery by a decrease in glucose production [66]. Recently, Cok et. al. compared the effects of isoflurane and propofol anaesthesia, both given in combination with remifentanil in forty patients undergoing craniotomy, on blood glucose, insulin and cortisol levels during surgery . A significant difference in the blood glucose values was found between the groups [67]. Propofol-based anaesthesia was more effective at stabilising the blood glucose level, especially after the first hour of operation whereas a more continuous rise in blood glucose levels were seen with isoflurane anaesthesia [67]. The insulin values at the 60th min of operation were significantly lower in the isoflurane group than in the propofol group [67]. Today, several experimental and clinical research works clearly demonstrate that isoflurane with or without surgery increases blood glucose level by inducing glucose production and decreasing glucose utilisation [68-71]. In another prospective randomized study of forty patients without DM, elective total abdominal hysterectomy were performed either with sevoflurane anaesthesia (SA) or total intravenous anaesthesia (TIVA) with propofol and remifentanil [72]. Plasma levels of adrenaline, noradrenaline, cortisol and glucose were significantly lower with TIVA than with SA, but there was no difference in IL-6 levels between the two groups [72]. Blood pressure and heart rate were also significantly lower with TIVA than with SA , but both anaesthesia techniques prevented increases in heart rate and blood pressure during the whole surgery [72]. In a clinical study encompassing 20 patients undergoing abdominal hysterectomy randomly assigned to receive either sevoflurane or isoflurane anaesthesia, isotope dilution technique did not show any differences in endogenous glucose production and plasma glucose clearance between these two types of anaesthesia [73]. Authors of the research work concluded that application of sevoflurane impaired glucose tolerance to the same degree as did isoflurane anaesthesia [73]. Thus, glucose intolerance during sevoflurane or isoflurane anaesthesia was independent of the type of inhalational agent and dosage up to 1.5 MAC [73]. In fifty patients undergoing coronary artery bypass grafting, stress-related reactions evaluated by plasma levels of cortisol and creatinine kinase musclebrain and haemodynamic responses were better controlled using propofol than desflurane inhalation added to a subanaesthetic dose of propofol [74]. Moreover, patients in the propofol group were extubated, on average, 2.3 hours earlier, they stayed for a shorter time in the intensive care unit, and they were discharged earlier to home when compared to those in desflurane group [74]. Summarising all of the above it seems that propofol in combination with opioids provides much better stability of the blood glucose level during anaesthesia compared to inhalation agents such as enflurane, isoflurane, sevoflurane and desflurane. In a clinical study with twenty healthy patients undergoing abdominal hysterectomy who received either continuous infusions of propofol supplemented with sufentanil, or enflurane anaesthesia, the intravenous anaesthesia group completely suppressed the intraoperative endocrine stress response and attenuated the increase in plasma glucose concentration [75]. However, in this study propofol/sufentanil anaesthesia did not inhibit the metabolic endocrine changes two hours after surgery and these were even more pronounced than after inhaled anaesthesia [75]. All studies mentioned above were performed in healthy patients and the application of inhaled agents in these research works did not result in severe hyperglycaemia (blood glucose level >10 mmol/L) [54-75]. We were only able to find one clinical retrospective study comparing the influence of sevoflurane or propofol anaesthesia on the incidence of hyperglycaemia in patients with DM2 undergoing lung surgery [76]. The authors of the study found that although blood glucose levels 2 hours after surgery were significantly lower in the propofol anaesthetised patients than in the patients with sevoflurane anaesthesia, there was no difference in the incidence of persistent hyperglycaemia during the perioperative period [76]. Experimental research work in rats demonstrated that anaesthesia with propofol enhances insulin secretion and concomitantly exaggerates insulin resistance, compared to anaesthesia by sevoflurane [77]. In experiments with pigs, sevoflurane, like other inhalation agents, activated adenosine triphosphate-sensitive potassium channels in β-islet cells, and reduced insulin secretion [78]. Apparently, propofol may have some advantages over inhalational agents in diabetic patients when strict peri-operative glycemic control is needed. Finally, two clinical prospective studies investigating the influence of different type of anaesthesia on the incidence of persistent hyperglycaemia during the peri-, post-operative period in diabetic patients are underway [79, 80].
Few studies have attempted to assess the influence of regional anaesthesia on the development of hyperglycaemia during the peri-, post-operative period. In a prospective, randomized controlled study of patients undergoing elective total hip replacement, spinal anaesthesia in contrast to general anaesthesia significantly reduced glucose levels in both non-diabetic and diabetic patients [81]. Another prospective comparative study of non-diabetic pregnant women who underwent elective caesarean section surgery, spinal anaesthesia also demonstrated significantly lower blood glucose concentrations compared to parturients under general anaesthesia [82]. Application of thoracic epidural anaesthesia combined with general anaesthesia improved glucose homeostasis for the 24 hours in low-risk patients undergoing cardiac bypass surgery [83]. In another study of low-risk patients scheduled for elective coronary artery bypass grafting with or without aortic valve replacement, high thoracic epidural anaesthesia (HTEA) also preserved glucose metabolism better and led to a lesser degree of stress-related hyperglycaemia in the postoperative period [85]. In this study the number of patients not receiving insulin in the postoperative period was significantly higher in the HTEA treated group [85]. In patients without DM undergoing either hip or knee arthroplasty, epidural anaesthesia decreased the incidence of insulin resistance for 48 hours after surgery only in patients who were insulin-resistant before surgery [84]. In a study on patients undergoing colorectal surgery, epidural blockade as an addition to general anaesthesia effectively attenuated both peri- and post-operative hyperglycaemia by modification of glucose production [86]. In healthy patients undergoing hysterectomy, normal glucose tolerance and insulin release were observed under epidural anaesthesia, whereas general anaesthesia produced decreases in both glucose tolerance and insulin release [87]. These studies seems to indicate that spinal or epidural anaesthesia, in contrast to general anaesthesia , may effectively attenuate the hyperglycaemic response to different types of surgery in both non-diabetic and diabetic patients. It has to be noted, however, that there still are no studies demonstrating that regional anaesthesia is superior to general anaesthesia in terms of mortality or major complications in surgical patients.
Precise planning of peri- and post-operative glucose management combined with detailed pre-operative evaluation of the presence of specific complications of DM (longterm hyperglycaemia) can significantly influence morbidity, length of hospital stay, and mortality in diabetic patients [53]. There are several important principles for the management of peri- and post-operative hyperglycaemia that anaesthesia personnel need to remember.
The first principle is that modification of insulin regimens and oral hypoglycaemic drugs is an essential component of the pre-operative preparation of diabetic patients. In March 2021, the Centre for Perioperative Care of the Royal College of Anaesthetists (UK) published a new «Guideline for Perioperative Care for People with Diabetes Mellitus Undergoing Elective and Emergency Surgery» that contains a detailed description of needful modifications of pre operative anti-diabetic medications [88]. In short, almost all oral hypoglycaemic drugs (except metformin) must be omitted on the day of surgery while special attention must be paid to insulin management (typically a 20–50% reduction in insulin daily dose in anticipation of missing one meal) [88]. In general, it is assumed that one unit of subcutaneous rapid acting analogue insulin will drop blood glucose by 3 mmol/l. However, wherever possible medical staff should take advice from the diabetic patient about the amount of insulin normally required to correct a high blood glucose [88]. Patients with insulin pump therapy require special attention. Insulin pumps infuse insulin through a small subcutaneous catheter and supply a baseline infusion of rapid acting insulin, with boluses self-administered as required by the patient. It seems that it is much safer to stop the pump in the peri-operative period, but in the case of day surgery and anticipation of missing one meal the use of insulin pumps intraoperatively is possible. In that case, capillary blood glucose (CBG) measurement should be taken regularly.
The second principle is that type I diabetics who are predisposed to DKA development, must be treated with a «basal dose of insulin» at all times unless the patient is hypoglycaemic (Fig. 5). Thus, insulin/glucose/potassium infusion must be continued until regular diet and insulin intake restart [88]. Different types of insulin/glucose mixture are proposed for this [89]. In our hospital we use 0,5 ml of Humalog 100 IE/ml which corresponds 50 IE added to 500 ml of 5% glucose thus creating the final concentration of 0,1 IE per ml of the mixture. Infusion rates of the mixture can be adjusted according to CBG measurement (Table 1). At the beginning of the infusion, CBG measurements should be taken hourly or in case of CBG instability even more frequently (10-15 min) to ensure that the intravenous infusion rate of the mixture is correct and that the patient is not hypoglycaemic.
Figure 5. Peri-operative effects of Insulin (inhibition and stimulation).
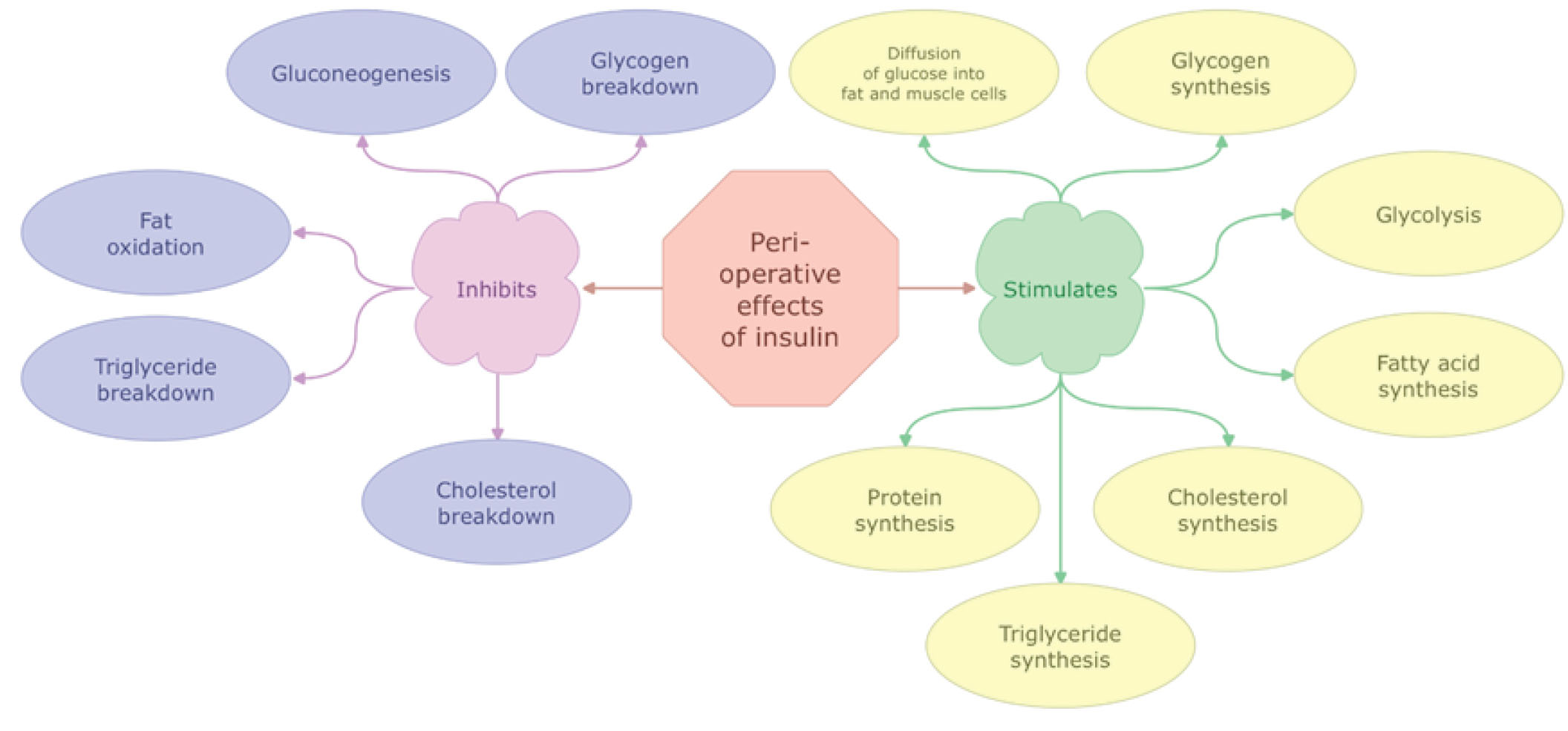
Table 1. Proposed infusion rate of 5% glucose with insulin 0.1 IE /ml according to capillary blood glucose measurement.
| Blood Glucose (mmol/l) | < 5 | 5-6.9 | 7-9.9 | 10-12.9 | 13-18 | > 18 |
| Infusion rate (ml/hour) | 0 | 10 | 15 | 20 | 30 | 40 |
The third principle is that the CBG target range should be at 6–10 mmol/l, although up to 12 mmol/l may be acceptable [88, 89]. The presence of ketones in blood and urine should be checked in case of several consecutive measurements of blood sugar > 12 mmol/l [88]. In case of capillary ketone > 3,0 mmol/l or urinary ketone 2+, pH < 7,3 or HCO3 < 15, DKA treatment should be applied. Patients treated with sodium-glucose cotransporter-2 inhibitors (SGLT2i) require special attention as they can develop non-classic “euglycaemic” DKA that may be accentuated during surgery [89]. Detection of euglycaemic DKA may be difficult during the peri-operative period due to near-normal blood glucose levels, especially if blood or urine ketone levels are not controlled [89]. Moreover, elderly patients treated with SGLT2i may be more prone to peri-operative hypovolemia and hypotension due to natriuresis and increased diuresis [89]. Treatment with SGLT2i can increase the risk of hypoglycaemia when they are used in conjunction with insulin [90,91]. Additionally, SGLT2i may influence electrolyte balance and provoke hyponatraemia and hyperkalemia, especially in patients with renal failure [90,91].
The fourth principle is that infusion of a higher concentration of insulin may be required in case of CBG instability and/or poorly controlled diabetes (defined as a HbA1c > 8,5%), emergency surgery, and for patients who will not return quickly to a normal diet and their usual diabetes regimen. In these clinical situations, we recommend the use of a syringe driver with 50 IE of Humalog or Actrapid in 49,5 ml of sodium chloride 0,9% (i.e. concentration of 1 IE per ml) that should be combined with i.v. infusion of 500 ml of 10% glucose, with 20 mmol KCl (unless K+>5 mmol/l), at 40 ml per hour via a volumetric pump. The insulin syringe driver usually starts with 1 IE per hour and then it is adjusted according to CBG measurement. Proposed infusion rates of this insulin mixture are presented in table 2. Both insulin and glucose infusions should run via the same intravenous cannula. A fresh insulin solution should be prepared every 24 hours for immediate use. Application of glucose infusion as a substrate is also needed to prevent proteolysis, lipolysis and ketogenesis. Iatrogenic hyponatraemia can be avoided with application of a mixture of 5% glucose and 0,45% saline.
Table 2. Proposed infusion rate of 0.9% saline with insulin 1 IE/ml according to capillary blood glucose measurement.
| Blood Glucose (mmol/l) | < 3 | 3-6 | 6-8 | 8-12 | 12-15 | > 15 |
| Infusion rate (IE/kg/hour) | 0 | 0.01 | 0.025 | 0.05 | 0.075 | 0.1 |
The fifth principle is that prolonged pre-operative fasting should be avoided in patients with DM [88]. Diabetic patients should always be scheduled to the beginning of the operation program and they should be prioritised in cases of both elective and emergency surgery.
The sixth principle is that diabetic patients should be managed as a day case if the surgical procedure is suitable for it and the patient fulfils the criteria for treatment in a day-case surgery department [88]. Well-controlled diabetes should not be a contra-indication to day-case surgery [88]. Not all patients require a variable rate intravenous insulin infusion. Diabetic patients undergoing a short surgical procedure and likely to miss only one meal can be managed by modifications of their medications [88].
The seventh principle is that HbA1C is a marker of longterm glycaemic control and pre-operative measurement of it may help to identify patients with long-term hyperglycaemia. In pre-operative evaluation, anaesthetists should recognise the clinical manifestations of diabetic autonomic neuropathy such as resting tachycardia, exercise intolerance, orthostatic hypotension, constipation, gastroparesis, sudomotor dysfunction and impaired neurovascular function. Diabetic stiff-joint syndrome and therefore difficult intubation may be an unexpected problem in the peri operative period [92,93]. Additionally, in these patients, gastrointestinal autonomic neuropathy with silent gastroparesis may result in gastric aspiration in the introduction phase of anaesthesia [94,95]. Diabetes mellitus is often associated with metabolic syndrome, which is characterised by four common findings: hyperglycaemia, hypertension, visceral obesity and dyslipidemia. Indeed, narrow and full pre-operative evaluation is needed for diabetic patients. Finally, no prospective study has shown that decreasing HbA1C pre-operatively to a certain level will improve outcomes. However, some guidelines of professional associations of anaesthetists recommend to postpone elective surgery in case of HbA1C > 8,5% for optimisation of glycaemic control [96].
Pathophysiological effects of long-term and/or stressrelated hyperglycaemia in DM patients may significantly influence morbidity, length of hospital stay, and mortality by different molecular and pathophysiological mechanisms. Scientific evidence indicates that application of anaesthesia with propofol may have advantages over inhalational agents in diabetic patients when strict peri-operative glycaemic control is needed, but this benefit seems to disappear postoperatively. There may also be some evidence that when propofol is discontinued, strict CBG measurements should be used because of a possible rebound effect. Meanwhile, two clinical trials comparing the influence of different anaesthetic agents on peri-and post-operative glycaemic status in diabetic patients are currently underway. Application of regional blockade as an addition to general anaesthesia effectively attenuates the hyperglycaemic response to different types of surgery in both non-diabetic and diabetic patients. For better management of peri- and post-operative hyperglycaemia in diabetic patients we have proposed several important practical principles in this review.
Disclosure. The authors declare that they have no competing interests.
Author contribution. Kuklin V.N., Matri J., Barlow N.P., Tveit S.H., Kvernberg J.E., Ringvold E.-M., Dahl V. - all authors according to the ICMJE criteria participated in the development of the concept of the article, obtaining and analyzing factual data, writing and editing the text of the article, checking and approving the text of the article.