
A critical illness is a complex of pathophysiological body changes that require the replacement of the functions of vital organs and systems in order to prevent the imminent death [1–3]. Modern medicine has accumulated enough facts to differentiate critical illness on an etiological basis. However, in pathogenetic terms, there is often a similarity in the manifestations of diseases that seem to be of different origin. As a rule critical illness is a pathology of all organs and systems, which are based on hypoxia. Differences can only consist of the fact that some functions, for example, respiratory or circulatory, need immediate and direct correction; the activity of other systems (renal, hepatic, gastrointestinal tract, endocrine system) can be optimized in a “critical” patient only with a satisfactory correction of the first two systems. However, compensation of circulatory or respiratory insufficiency does not always lead to the resolution of deep metabolic disorders in the body resulting from deoxia [3].
The neuroendocrine system plays the most important role in the shaping the development of physiological, and subsequently pathological, reactivity of the organism in critical illness. The concept of the neuroendocrine system includes multiple connections between the endocrine and central nervous systems, their relation in the control of homeostasis, as well as in the formation of a response to the effects of various physical stressors [4].
At the same time, the stimulation of the immune system by means of foreign pathogens leads to a complex neuroimmunoendocrine interaction in order to avoid the development of critical illness. This interaction is formed through the integration of the influx of information from the vagus nerve, peripheral cytokine interactions with the receptors in the area of organs surrounding the ventricles of the brain, cerebral vessels and local formation of cytokines within the central nervous system. This process leads to a complex neuroimmunoendocrine response during the development of critical illness [5].
Téblick A. in article from 2019 divides the changes in the pituitary-adrenal system in critical illness into three phases. An increase of the adrenocorticotropic hormone (ACTH) level and, as a result, cortisol in blood plasma during the acute phase of critical illness is characterized by a stress response [6]. If vital functions are not restored within a few days, the development of the syndrome of multiple organ failure of the critical illness passes from the acute phase to the subacute (up to 14–21 days), and then to the chronic phase (more than 14–21 days). In the subacute and chronic phases of the critical illness, a multidirectional level of blood cortisol is observed against the background of a suppressed level of ACTH [7] (Fig. 1). At the same time, an increasing level of cortisol is associated with a lethal outcome [6].

There are the fluctuations in ACTH levels and the multidirectional level of cortisol in the blood plasma in different phases of the critical illness that formed the basis of the concept of сritical illness-related corticosteroid insufficiency (CIRCI). CIRCI is a dysregulation at any level in the hypothalamus-pituitary-adrenals-target tissue system, which leads to a decrease in the production of cortisol by the adrenal glands and/or tissue resistance to glucocorticoids, which in turn is manifested by a high level of cortisol in the blood plasma [8].
Further development of the critical illness because of the various pathophysiological changes in patients leads to the formation of multiple organ failure. In the intensive care of critical illness and multiple organ failure, various methods of treatment are used, among which there are highly aggressive ones that can, and both directly and indirectly, have an independent additional effect on the functioning of organs and systems, including the endocrine system.
Extracorporeal membrane oxygenation (ECMO) is a temporary highly aggressive method of life support in critical illness, primarily associated with the development of acute severe respiratory and/or heart failure. ECMO is not a fully therapeutic measure, but only an organ replacement measure that gives time to maintain adequate blood circulation, oxygenation of organs and tissues, and conduct pathogenetically substantiated treatment [9]. At the same time, ECMO is capable of exerting an independent additional negative impact on the functioning of organs and systems through the hyperoxia in veno-arterial ECMO, the development of a cascade of inflammatory reactions (coagulopathy, increased levels of cytokines, the compliment system, endothelial dysfunction, traumatization of erythrocytes) when blood comes into contact with an artificial circulation circuit [10–12].
Nowadays, there are no data on to changes in the pituitary-adrenal system in patients undergoing ECMO. Several studies consider the use of VA-ECMO in the catecholamine shock caused by the pheochromocytoma and thyroid storm, as well as heart failure caused by diabetic ketoacidosis [13, 14]. The analysis of these studies is not presented in this article, since the patients initially had endocrine disorders that required the initiation of ECMO.
The aim of our study is to evaluate the relationship between plasma levels of ACTH and the cortisol in critically ill patients undergoing ECMO.
A prospective single center study included 47 patients requiring ECMO. The connection to ECMO took place both at the stage of the primary hospital with a subsequent transportation of patients to the ECMO center, and in the intensive care unit at the ECMO center, where they were further treated.
This study was approved by the ethics committee of State Scientific Center of A.I. Burnazyan Federal Medical Biophysical Center of the Federal Medical and Biological Agency of Russia (protocol No. 9 dated April 25, 2016). Inclusion Criteria: Patients over 18 years old who are undergoing ECMO. Exclusion criteria: pregnancy, brain death, use of synthetic glucocorticoids, history of thyroid and adrenal diseases. Patients were monitored during ECMO in the intensive care units (ICU) A.I. Burnazyan FMBA of Russia.
The period of observation of patients began from the moment of initiation of ECMO and it was carried out until weaning from it. Demographic characteristics are represented by descriptive statistics. The anamnesis was collected according to the medical documentation; the moment of initiation and the duration of mechanical ventilation before the start of ECMO were also recorded. The clinical and neurological examination included an assessment of the patient's condition using the following scales: Acute Physiology and Chronic Health Evaluation (APACHE II), Sequential Organ Failure Assessment (SOFA).
Starting criteria for veno-venous ECMO: respiratory index < 50 mm Hg. Art. for > 3 hours; respiratory index < 80 mm Hg. Art. for > 6 hours; arterial pH < 7.25 with hypercapnia > 60 mm Hg. for > 6 hours against the background of an increased frequency of respiratory movements up to 35 times per minute [15–17]. In order to maintain the blood circulation in refractory cardiogenic shock (systolic blood pressure below 90 mm Hg and signs of severe organ hypoperfusion — impaired mental status, urine output below 30 ml/hour, cold extremities, pulmonary edema, use and ineffectiveness of inotropes, vasodilators, intra-aortic balloon pump) used VA-ECMO [18, 19].
When the saturation of arterial blood is above 95 %, the carbon dioxide voltage is less than 50 mm Hg, at an oxygen fraction level of not more than 50 % and with a performance of veno-venous ECMO close to the patient's cardiac output, a transition was made to the basic algorithm for weaning the patient from ECMO [20, 21]. Weaning from venoarterial ECMO was based on the following parameters: assessment of the patient's status, dose of inotropic and vasopressor support, resolution of organ dysfunction, respiratory index > 100 % [22].
Laboratory studies included blood sampling on the zero day (D0), on the day of the start of ECMO, then the first, third, fifth, seventh days (D1, D3, D5, D7) and then every second day until weaning from ECMO/death on ECMO. The pituitary-adrenal axis was assessed (total cortisol, ACTH). Plasma ACTH levels were determined by the chemiluminescent enzyme immunoassay. The content of cortisol in the blood serum was determined by immunochemiluminescent analysis. The time of blood sampling for ACTH and total cortisol for DO depended on the time of ECMO initiation. A follow-up hormonal assessment was performed before 8:00 a.m. from a central venous catheter.
As part of the study, it was decided to focus on the existing reference values of ACTH and total cortisol in blood plasma. The identified differences were indicated upon receipt of statistically significant results in the range of reference values.
In patients on ECMO, the following signs were monitored: saturation, partial pressure of oxygen and carbon dioxide, lactate level in blood plasma, electrolyte composition of blood, acid-base balance. Blood sampling for monitoring the listed parameters was carried out from 4 places: sampling cannula; after oxygenator; from a central venous catheter; and from the patient's arterial blood.
CIRCI has been considered in patients with norepinephrine requirements greater than 0.25 µg/kg/min. Intravenous hormone replacement therapy with hydrocortisone was administered to patients who maintained a need for norepinephrine of 0.25 μg/kg/min or more for more than 24 hours, despite the achievement of normovolemia, in order to maintain a systolic blood pressure of 90 mm Hg. Art. and above or mean arterial pressure of 65 mm Hg. Art. and higher. An initial dose of hydrocortisone on the first day was 300 mg (100 mg, intravenous bolus, then 50 mg 4 times per knock, bolus), the second and subsequent knocks — 200 mg, 4 times a day (06:00; 12:00; 18:00; 24:00) [23]. A dose of hydrocortisone administered intravenously was reduced by 25–50 mg per day, starting from 00:00. A reduction in the daily dose of hydrocortisone was carried out after the complete cancellation of the dose of noradrenaline, with the subsequent transfer of the patient to hydrocortisone in the tablet form if necessary..
Statistical data processing was performed in the IBM SPSS Statistics program. Descriptive statistics methods were used to assess the study groups, (Me — median, Q1 — the first quartile and Q3 — the third quartile). The reliability of the data was determined by nonparametric criteria. To check the normality of the sample, the Shapiro-Wilk and Kolmogorov-Smirnov tests were used. Under the normal distribution of the studied indicator, a parametric criterion was used. Given that most of the indicators did not follow a normal distribution, we used nonparametric criteria. An intragroup correlation of traits was assessed by the Spearman's rank correlation coefficient (rs). Significance of differences between two unrelated groups was assessed by the Mann-Whitney test (U). The Wilcoxon test (W) was used to estimate the change in the parameter over time for two related samples. According to ROC-curves, the diagnostic significance of the identified intergroup differences was compared with the calculation of sensitivity and specificity. A critical significance upon examination the null hypothesis, was taken to be ≤ 0.05.
The study included 47 patients (Table 1). The indications for ECMO were: acute respiratory failure not corrected by mechanical ventilation — 40 (85 %); acute cardiovascular failure refractory to treatment with vasopressor and inotropic drugs, (circulatory failure) — 7 (15 %). Causes of acute respiratory failure are presented: bacterial and viral pneumonia — 9 (19 %); bacterial pneumonia — 25 (53 %); viral pneumonia — 6 (13 %); bacterial myocarditis 3 (7 %); acute myocardial infarction — 2 (4 %); cardiac arrest — 2 (4 %).
Patients who died — 29/47 (62 %): septic shock — 22/29 (76 %); pulmonary embolism — 2/29 (7 %); cardiogenic shock — 3/29 (10 %); hemorrhagic complications — 1/29 (3.5 %); brain death — 1/29 (3.5 %).
“ECMO weaning” is a procedure when the ECMO system is removed due to restoration of lung gas exchange and/or cardiac function. Further, these patients could survive or die at any stage after weaning from ECMO. Lethal outcome during ECMO we designated “death on ECMO”.
Table 1. Common characteristics between survivors and non-survivors patients
| Parameters | Survivors, n = 18 | Non-survivors, n = 29 | p |
|---|---|---|---|
| Age. Ме (25–75 %) | 40.5 (32–46) | 50 (35–60) | 0.039 |
| Sex m/f | 29/19 | ||
| Body mass index, kg/m2 | 27.6 (24.1–28) | 26.4(25.7–30.7) | 0.56 |
| SOFA — in day of ECMO weaning/death on ECMO | 8 (5–10.5) | 12 (12–16) | 0.001 |
| SOFA — in first day of observation | 9 (5.7–12) | 12 (8.5–13) | 0.18 |
| APACHE II in day of ECMO connection | 19.5 (18.7–22) | 24 (20–31) | 0.01 |
| From the moment of connection to the meconial ventilator, before connecting to ECMO, days | 3 (1–6) | 3 (1–4) | 0.5 |
| ICU stay, days | 17 (14.2–25.2) | 15 (5.5–19.5) | 0.034 |
| ECMO duration, days | 7.5 (5.7–10) | 8 (5–15.5) | 0.594 |
| Duration of therapeutic normothermia on ECMO, days | 2 (2–3) | 3 (3–9) | 0.049 |
| The level of lactate in arterial blood on the first day of connection to ECMO (mmol/l), n = 47 | 2.9 (2.2–4.4) | ||
| The level of lactate in arterial blood on the first day of connection to ECMO (mmol/l) | 2.8 (2.1–4.4) | 3 (2.2–4.6) | 0.9 |
| Arterial lactate level on day of weaning/death on ECMO (mmol/L) | 0.9 (0.6–1.2) | 3.8 (2.1–7) | 0.001 |
| Medications for general anesthesia and sedation | Propofol, thiopental, fentanyl, dexmedetomedin | ||
| Peripheral ECMO Veno-venous/veno-arterial, n |
40/7 | ||
| Diseases that lead to the development of a critical illness and connecting ECMO: | |||
| Pneumonia | 40 | ||
| Myocarditis | 1 | ||
| Acute myocardial infarction complicated with cardiac arrest | 6 | ||
According to the results of assessing the patients’ condition at the time of admission to the ICU on the APACHE II scale, the number of points was higher in non-survivors patients. An older age group was represented by non-survivors patients. A length of ventilator stay did not differ between the two groups. On the last day of observation during ECMO, the SOFA score was higher in non-survivors patients. Patients with a favorable outcome in the ICU took longer to arrive than those who died. There was no statistically significant difference between survivors and non-survivors patients depending on the length of mechanical ventilation prior to ECMO.
Arterial lactate levels on the first day of ECMO follow-up did not differ significantly between survivors / non-survivors patients. The total lactate level was 2.9 (2.2–4.4) mmol/l. At weaning/death on ECMO, lactate levels were higher in non-survivors patients.
The results of the cortisol level in blood plasma depending on the time between survivors and non-survivors patients (Me (Q1–Q3)) are presented (Fig. 2). On the day of ECMO initiation, on the first day and then every second day, the average level of total cortisol was D0 between survivors and deaths (406–332; p = 0.52); D1 (237–428; p = 0.24); D3 (433–636; p = 0.05), D5 (729–772; p = 0.29); D7 (439–1063; p = 0.03), D9 (862–778; p = 0.42), D11 (319–699; p = 0.23), D13 (652–789; p = 0.05), the last day of observation (475–1474; p = 0.001), respectively. The cortisol level in the blood was higher in non-survivors patients on D3, D7, D13 and on the last day of observation. Reference values of the total cortisol level were (171–536 nmol/l).

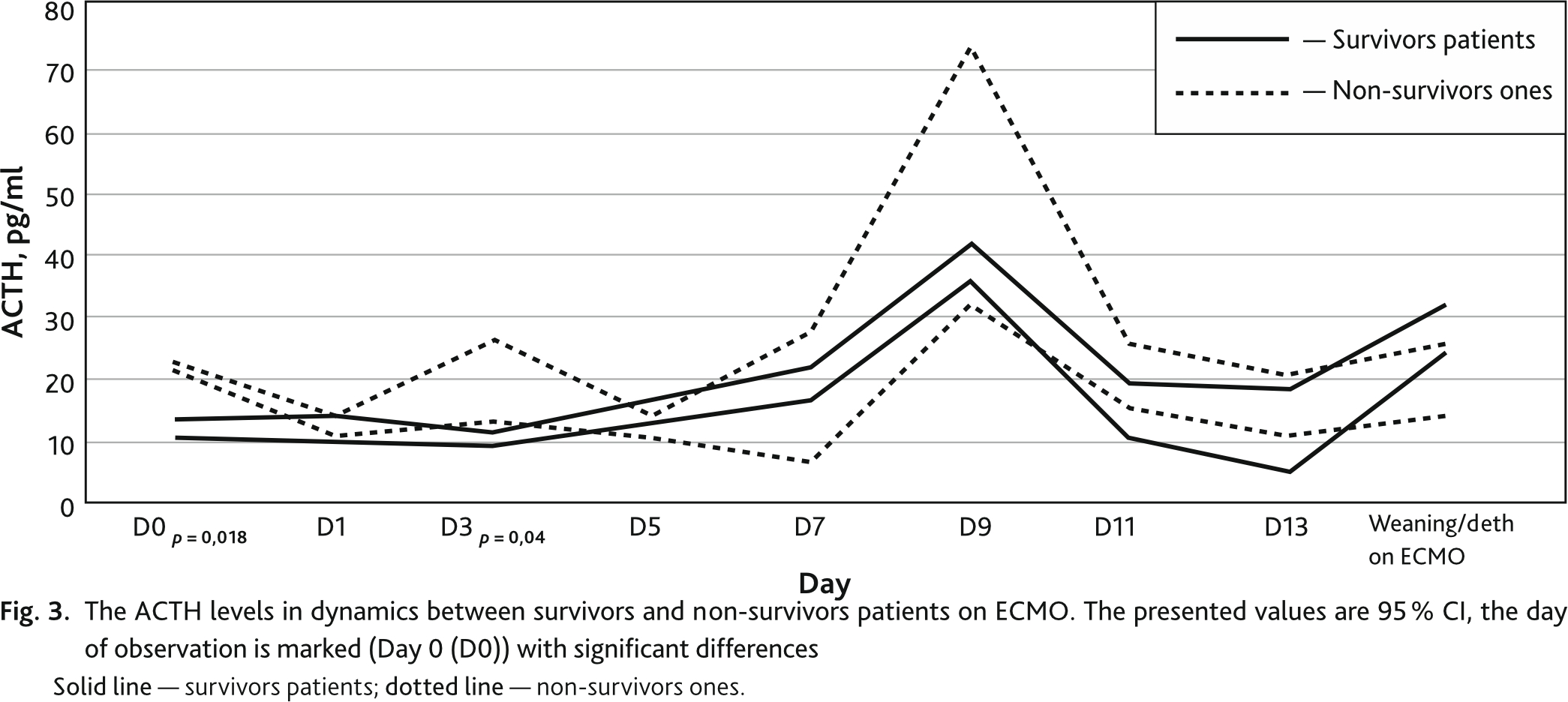
In addition, there were differences in ACTH levels between groups of non-survivors and survivors patients on ECMO (Fig. 3). On the day of ECMO initiation, on the first day and then every second day, the average ACTH level between survivors and deaths was D0 (12–22; р = 0.018); D1 (12.0–13.5; p = 0.36); D3 (10.3–19.6; p = 0.04), D5 (16.8–12.0; p = 0.47); D7 (19.8–17.0; p = 0.98), D9 (38.8–73.0; p = 0.19), D11 (14.8–25.4; p = 0.29), D13 (11.7–15.8; p = 0.87) respectively, weaning/death on ECMO (28.2–20.0; p = 0.68), respectively. The ACTH level in the blood was higher in the non-survivors patients on the day of the ECMO start and on D3 follow-up. On D9, there was an increase in ACTH levels in non-survivors patients, but without statistically significant differences. Reference values of ACTH level were (< 46 pg/ml).
An assessment of the changes’ dynamics in the cortisol level in the blood plasma was performed between non-survivors and survivors patients during treatment with hydrocortisone (29/47) at a low dose (300 mg on the first day, then 200 mg daily) with a need for norepinephrine 0.25 μg/kg/min. more than 24 hours. The cortisol levels on D7, (358 (333–407) — 1056 (757–1474)) p = 0.001 and on the day of weaning/death on ECMO (358 (252–400) — 1942 (1128–2375)) p = 0.001 was higher in the dead.
We also evaluated the change in cortisol levels over time during ECMO in the group of both non-survivors and survivors patients during treatment with hydrocortisone. Analysis of the repeated changes showed an increase in cortisol levels only in patients who died, despite ongoing treatment with hydrocortisone (Me (Q1–Q3)), D0 (381 (241–738)); D1 (512 (191–1627)); D3 (757 (401–1624)); D5 (838 (447–1578)); D7 (1056 (757–1474)); D9 (778 (373–1515)); D11 (922 (699–2048)); D13 (1162 (701–2542)); ECMO weaning/death (1942 (1128–2375)); χ2 = 15.9; p = 0.026.
Comparing cortisol levels between those who died with (19/29) and those without (10/18) hydrocortisone treatment on the last day of ECMO follow-up, the cortisol levels were higher in the hydrocortisone group 1942 (1128–2375) — 1320 (659–1474), p = 0.001, respectively.
The cortisol levels were correlated with SOFA scores on the day of weaning/death on ECMO. With increasing values of the SOFA scale, a statistically significant increase in the cortisol level in the blood plasma was observed, r = 0.29, p = 0.04.
While correlation analyzing of the C-reactive protein level and cortisol in blood plasma on the day of weaning/death on ECMO, their positive relationship was observed, r = 0.37, p = 0.016.
There was a positive correlation between lactate and cortisol levels on weaning/death day on ECMO, r = 0.6 at p = 0.001.
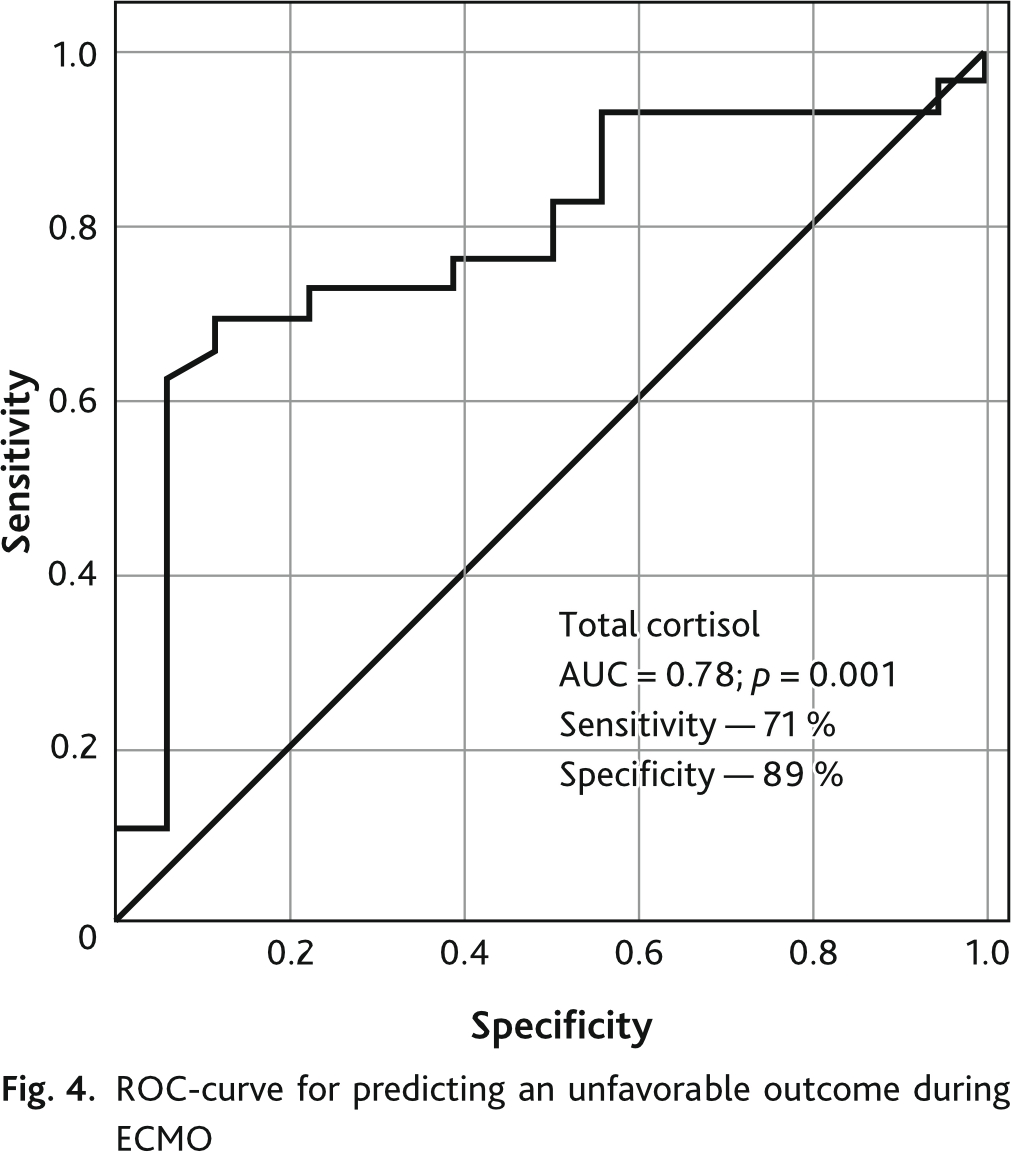
Analysis of the ROC-curve showed that the total cortisol level in blood plasma demonstrates a sensitivity of 71 % and a specificity of 89 % in relation to an adverse outcome during ECMO (Fig. 4).
Against the background of the development of multiple protective neuroimmunoendocrine reactions in the event of critical illness, a systemic inflammatory response syndrome is formed [3, 4]. A trigger mechanism for the development of the systemic inflammatory response syndrome in critical illness is an increase in the level of a molecular fragment associated with damage and/or an increase in the level of a pathogen-associated molecular fragment [24, 25]. Under such conditions, the limbic system recognizes the danger, analyzes it, compares it with past experience and chooses a way to overcome the critical illness. At the same time, the hypothalamic and noradrenergic systems directly modulate metabolic, immune and hemodynamic responses [26–28].
One of these modulations is the activation of the hypothalamic-pituitary-adrenal system by pro-inflammatory cytokines and afferent pathways of the vagus nerve [28–30]. As a result, an increase in the secretion of glucocorticoids has a powerful anti-inflammatory effect reducing the transcription of cytokines due to the suppression of the nuclear factor kappa-bi, a universal transcription factor that controls the expression of immune response, apoptosis, and cell cycle genes [5, 26, 31, 32]. A sympathetic link of the autonomic nervous system also leads to a decrease in the release of pro-inflammatory cytokines while the synthesis of anti-inflammatory cytokines remains the same [28].
If the vital functions of the body are not restored within a few days, the critical illness passes from the acute to the subacute phase [7]. A development and formation of the subacute phase of critical illness is based on the increasing systemic level of pro-inflammatory cytokines, hypoxic-ischemic brain damage, pathological permeability of capillary endothelial cells that form the blood-brain barrier and, as a result, the penetration of pro-inflammatory mediators and other neurotoxic molecules into the brain [33]. Circulating pro-inflammatory cytokines, penetrating through the blood-brain barrier, damage the nuclei of the limbic, hypothalamic and noradrenergic systems that leads to disruption of the neuroendocrine response in critical illness with the formation of organ dysfunction [34].
It is during the subacute phase of critical illness that a multidirectional cortisol level in the blood plasma is observed against the background of the most often the suppressed ACTH level [35], which can be explained by several factors: a stimulation of cortisol in the adrenal glands by pro-inflammatory cytokines [36]; a decreased activity of cortisol-metabolizing enzymes [6]; corticoresistance caused by inhibition of glucocorticoid α receptors which are classical glucocorticoid receptors and function as a ligand-dependent transcription factor [37].
Nonspecific symptoms of CIRCI include nausea, vomiting, fluid-resistant and catecholamine-resistant hypotension, hyponatremia, hyperkalemia, hypoglycemia, eosinophilia, lymphocytosis and fever. Neurological disorders can manifest as delirium and coma [38]. It should be noted that hyponatremia is not always observed in adrenal dysfunction since the use of drugs such as sodium bicarbonate, hyperosmolar solutions, corrects the true electrolyte imbalance.
The existing diagnostic criteria for assessing “primary adrenal insufficiency” cannot be considered in critically ill patients for several reasons: a test with a high dose of synthetic ACTH is not a natural condition for the body especially with the development of sepsis, septic shock; lack of information on the response of the hypothalamic-pituitary-adrenal axis to stimuli such as hypotension; in critical illness a high level of cortisol is not an indicator of the safety of the hypothalamus–pituitary–adrenals–target tissue system since glucocorticoid resistance is not excluded [8, 39, 40].
A high plasma cortisol level indicates the highest probability of lethal outcome in the short-term follow-up period in critically ill patients [41–44]. In our study, a high plasma cortisol on the day of weaning/death on ECMO is also a specific and sensitive indicator of poor outcome.
In our work, we observed regularity in the flow of critical illness phases during ECMO. On the one hand the level of hormones of the pituitary-adrenal system in patients on ECMO, on the one hand, showed significant dynamics over time. On the other hand, it differed in the groups of survivors and non-survivors patients.
An increase in the ACTH level in the blood plasma in the range of reference values was observed on the zero day in the group who subsequently died compared with the survivors. This result can be considered as a stress response of the body to the connection of a highly aggressive treatment method, which is an independent factor in the development of a cascade of inflammatory reactions [10]. It is likely that the increase in the ACTH levels in non-survivors patients is due to both the degree of organ damage and the severity of adrenal dysfunction.
During the dynamic assessment of the level of cortisol in the blood plasma, its values were within the reference intervals from the moment of ECMO connection. On the third day of ECMO, an increase in plasma cortisol levels was observed in patients who subsequently died, as opposed to later survivors. On the third day of ECMO, exactly the same changes were noted in the ACTH level. In the group of patients who subsequently died, the ACTH level was higher than in the survivors’ group but within the reference values. Far more significant differences in the cortisol levels were observed by 7th day while the average cortisol values remained within the reference values in survivors patients, while a significant excess of the reference values was observed in non-survivors patients. Starting from the 7th day, the groups of survivors and non-survivors patients showed an increase in the ACTH level that reaches its maximum on the 9th day. In response to an increase in the ACTH level, an increase in the concentration of cortisol in the blood plasma was noted in both groups by the 13th day of ECMO. While in the case of the survivors we can talk about the normalization of cortisol levels by day 13 of ECMO, in the group of the non-survivors patients there was a rapid increase in the cortisol levels significantly exceeding the reference values. And that this growth was observed until the onset of death. On the last day of observation, differences in cortisol levels became the most significant.
The period from the moment the patient is put on connected to the ventilator to the start of ECMO changes took from 1 to 6 days, which corresponds to the period of the acute phase of critical illness. There is no statistically significant difference between survivors and non-survivors patients depending on the length of mechanical ventilation prior to ECMO. Therefore, these results did not affect the outcome of the disease.
The concept of the critical illness’ phases is applicable and observed during ECMO. To date, it is impossible to identify clear boundaries between the transition of the acute phase to the subacute one. Based on fluctuations in plasma cortisol levels and ACTH, depletion of body reserves has been observed in potentially fatal patients.
So, from the third day we see a decrease in the cortisol level in the blood plasma in the survivors’ group — the transition of the acute phase of the critical illness to the subacute one. After weaning from ECMO, survivors patients should be viewed in terms of the course of the chronic phase of critical illness to subsequent recovery.
In subsequently non-survivors patients, there was no decrease in plasma cortisol levels, on the contrary, an increase. These changes are typical for the formation of the subacute stage of critical illness and may be an early predictor of an unfavorable outcome and emerging corticosteroid resistance [45, 46].
In the interval of the seventh — ninth days, depletion of the adrenal cortex is observed with a characteristic sharp surge in the ACTH level on the 9th day, observed both in survivors and in the dead. Due to the small number of remaining patients on ECMO, by the 9th day of observation, significant differences in the ACTH level in the blood plasma of the non-survivors and survivors patients were not detected, however, there was a trend towards higher ACTH values in the group of the non-survivors.
On the 13th day of observation, the cortisol level in blood plasma increased progressively in the group of non-survivors patients. The maximum differences in the cortisol levels in non-survivors and survivors patients were detected on the last day of observation.
An increase in the cortisol level in the blood plasma is typical within the reference values for patients who survived after an increase in the level of ACTH and, accordingly, stimulation of the adrenal glands during the course of the subacute phase of the critical illness (on day 9). At the same time, a different development was observed in the dead: a significant increase in the level of ACTH was accompanied by a progressive increase in the cortisol level in the blood plasma by day 13, with its subsequent increase until the onset of death.
Based on the data obtained within the framework of the study and the results of third-party studies, we concluded that in the group of non-survivors patients, the phenomenon of corticosteroid resistance was observed. Dysregulation of systemic inflammation in the form of excessive transcription of cytokines due to excessive activation of the nuclear factor “kappa-bi”, and as a result, suppression of the activity of the glucocorticoid receptor α, activated by endogenous or exogenous glucocorticoids, is an important pathogenetic mechanism of dysfunction of the lungs and other organs in patients with acute respiratory distress syndrome [47–52]. Analyzing the positive effect of exogenous glucocorticoids (natural and synthetic) in acute respiratory distress syndrome in the form of a decrease in the levels of inflammatory markers through the suppression of the nuclear factor kappa-light-chain-enhancer of activated B cells, we assume that the basis for the increase in the level of endogenous cortisol is the resistance of intracellular receptors of target tissues to him [55]. The study founds no difference between the level of C-reactive protein in survivors and non-survivors patients, which is probably related to the use and degree of the treatment’s aggressiveness.
The obtained positive relationship between C-reactive protein levels and plasma cortisol levels on the day of weaning/death on ECMO may support arguments in defense of the theory of the cortico-resistance’s development with an increase in inflammatory markers in critical illness patients.
A positive correlation between cortisol levels and SOFA scores on the day of weaning/death on ECMO showed high cortisol levels as an independent predictor of mortality.
One of the important indicators of generalized tissue hypoxia is such a surrogate marker as lactate level [54]. The high plasma cortisol levels and its positive correlation with arterial lactate levels in non-survivors patients on the day of weaning/death on ECMO also indicate cortisol reactivity in systemic tissue hypoxia [55]. An increase in plasma cortisol, along with lactate, is a predictor of poor outcome.
An increase in the cortisol levels in non-survivors patients both with and without hydrocortisone treatment most likely remains a criterion for poor outcome. Higher plasma cortisol levels were found in non-survivors patients treated with hydrocortisone; at the same time, it should be emphasized that the severity of the condition of these patients according to the SOFA scale was above 10 points.
Consideration of the acute and subacute phases of the critical illness during ECMO based on the analysis of fluctuations in the ACTH level and cortisol in the blood plasma allows us to speak about the development of adrenal dysfunction caused by a critical condition. Within the framework of the NDVKS concept, the following
Disclosure. The authors declare that they have no competing interests.
Author contribution. All authors according to the ICMJE criteria participated in the development of the concept of the article, obtaining and analyzing factual data, writing and editing the text of the article, checking and approving the text of the article.
Ethics approval. This study was approved by the ethics committee of State Scientific Center of A.I. Burnazyan Federal Medical Biophysical Center of the Federal Medical and Biological Agency of Russia (reference number: 9 dated April 25, 2016).
Acknowledgments. The authors would like to thank Greet Van den Berghe for kind permission to use images from his article in this paper.