
The successes of modern anesthesiology have significantly reduced the risk of anesthesia compared to the last century. However, the level of perioperative hospital mortality after elective surgery currently averages about 0.5 % [1]. It is estimated that more than 313 million adults worldwide undergo surgical interventions annually [2]. Thus, the number of deaths may result in several million deaths each year worldwide. Nevertheless, the study of the risk of death is associated with certain difficulties, since over the past half century this indicator has decreased a hundred times and studies involving an elusive number of subjects are required for the study.
Current research focuses on other outcome criteria – significant and minor postoperative complications. Thus, anesthetic risk is most often understood as the risk of postoperative complications. The rate of these complications varies in a wide range, ranging from 3 to 18 % [3–5]. The differences in the data are explained by the lack of clear definitions and differences in the design of studies, but the fact that the development of a postoperative complication increases the risk of death several times can be considered indisputable [1]. However, despite the importance of this issue, there is no clear idea in the modern literature about what is a high risk and which of the patients corresponds to this category.
Understanding whether a patient belongs to the high-risk category is an extremely important task — it allows you to obtain meaningful informed consent of the patient, as well as to understand whether strategies for preventing complications should be applied (goal-directed fluid therapy, protective mechanical ventilation, features of postoperative care, etc.).
Attempts of creating preoperative risk stratification tool have been made for many decades, some scales assess the initial physical status (ASA scale) [6, 7] and predict mortality, others assess the risk of specific complications (Revised cardiac index Lee, respiratory risk scale, etc.) [8, 9].
Scales that include intraoperative and postoperative indicators, such as a series of POSSUM scales are also being developed [10]. The analysis shows that in routine clinical practice, these scales are not used very often, which is due to their limitations: subjectivity, technical complexity and often low specificity and sensitivity.
Concomitant diseases are the strongest predictors of postoperative adverse events and mortality. It has been demonstrated that the Charlson comorbidity index of 3 or more increases the risk of death by 16 times within a year after surgery [11]. In addition, in most clinical studies, the ASA classification of physical status as a kind of comprehensive assessment of patients' comorbidity has repeatedly proved to be one of the strongest independent predictors of postoperative morbidity and mortality, despite the fact that this assessment is based on subjective perception [12, 13].
The main concomitant diseases that are independent predictors of perioperative complications are diseases of the cardiovascular and respiratory systems [14]. Increasing age, anemia, obesity, diabetes mellitus – these conditions also increase the risk of an unfavorable outcome [15–18]. Diseases of the central nervous system and neuromuscular diseases significantly disrupt the function of respiratory system, can change the level of autonomous regulation of the cardiovascular system, lead to significant cognitive disorders and nutritional insufficiency, which also increases the risk of perioperative complications [19].
On the other hand, in large-scale observational studies of recent years performed in a number of foreign countries, concomitant diseases were not identified as independent predictors of the development of postoperative complications [5], and preoperative assessment systems based on concomitant diseases, such as POSPOM, demonstrate contradictory prognostic value in non-cardiac surgery — from underestimating the risk of death [20] to its overestimation [21].
Thus, data on the impact of concomitant diseases on perioperative risk are contradictory and may be influenced by differences in the frequency and structure of these diseases in heterogeneous populations, as well as in different treatment strategies for cardiovascular, respiratory and other diseases. Identification of these risk factors is necessary to understand the pathophysiology of complications and to identify potential ways to reduce the anesthetic risk, such as correction of concomitant disease. Conducting a national study on the identification of risk factors for an unfavorable outcome is the first integral step towards creating a set of measures to improve the quality of perioperative care and reduce mortality [22].
Perioperative risk, of course, depends not only on the presence of concomitant diseases and their combinations, but also on the severity of surgical trauma [1, 23], as well as on the level of exposure to drugs for anesthesia and anesthetic techniques [5], therefore, risk stratification without taking into account of these factors also does not seem appropriate.
Objective. The aim of this study is to increase the accuracy of stratification of patients with high surgical and anesthetic risk in abdominal surgery and to identify ways to prevent postoperative complications.
It is planned to conduct a national multicenter prospective observational study using a questionnaire (see appendix 1) and a computer database developed on its basis.
The study has passed the registration procedure in the database ClinicalTrials.gov (The protocol of the study is available on the website ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT03945968)
Dates of the study: 01.06.2019–31.05.2024.
Inclusion criteria: patients over 18 years of age undergoing elective abdominal surgery, whose physical status corresponds to Class I–III according to ASA-PS classification.
Exclusion criteria: inability to assess the factors included in the questionnaire, acute massive blood loss, aspiration, bronchospasm, anaphylactic reactions, malignant hyperthermia, transurethral and transvaginal surgery, interventions on peripheral vessels and heart, thoracic operations, neck and head surgery, trauma surgery.

Fig. 1. Scheme of the study protocol
The sample size was calculated taking into account the fact that at least 10 cases of postoperative complications per factor included in the final regression model are required. Taking into account the wide range of the frequency of complications in previous studies (from 3 % to 20 %), we selected a lower bound for a more accurate assessment. To include 20 potential risk factors into the regression model, 200 cases of postoperative complications are required. The rate if complication is approximately 3 %, so we have to recruit at least 7000 participants. To take into account the risk of data loss, and to assess all potential risk factors, the size of the required sample was increased to 12,000 people, which will also allow us to assess the contribution of concomitant diseases to individual groups of complications. An additional 4000 will be recruited to validate the predictive model. The inclusion of the patient in the main and validated group will be carried out randomly.
The distribution of the studied parameters will be evaluated using the Kogmogorov—Smirnov test. Continuous data will be presented as median and interquartile range for normal distribution and as mean and standard deviation for nonparametric distribution. Categorical variables will be presented in the number of patients and the percentage.
For the initial assessment of the association of the factor with postoperative complications, a single-factor analysis will be performed using the χ2 test and the Mann—Whitney test. All variables with a significant relationship identified in the univariate analysis (p < 0.05) will be included in the logistic regression if there is no colinearity between them (correlation coefficient less than 0.25). The logistic regression model will be carried out using the step-by-step inclusion method in which the presence of a complication will be a dependent variable. Potential predictors will be removed if this exception does not lead to a significant change in the likelihood ratio. The criterion for excluding the factor will be set at a significance level of 0.05. Adjusted odds ratios and 95 % confidence intervals will also be calculated.
The obtained predictive model will be evaluated in a validated group using ROC analysis and the Hosmer—Lemeshov test.
The recruitment of patients to the study was started in July 2019, currently, at the end of June 2022, 6689 patients were included in the study (Fig. 2).
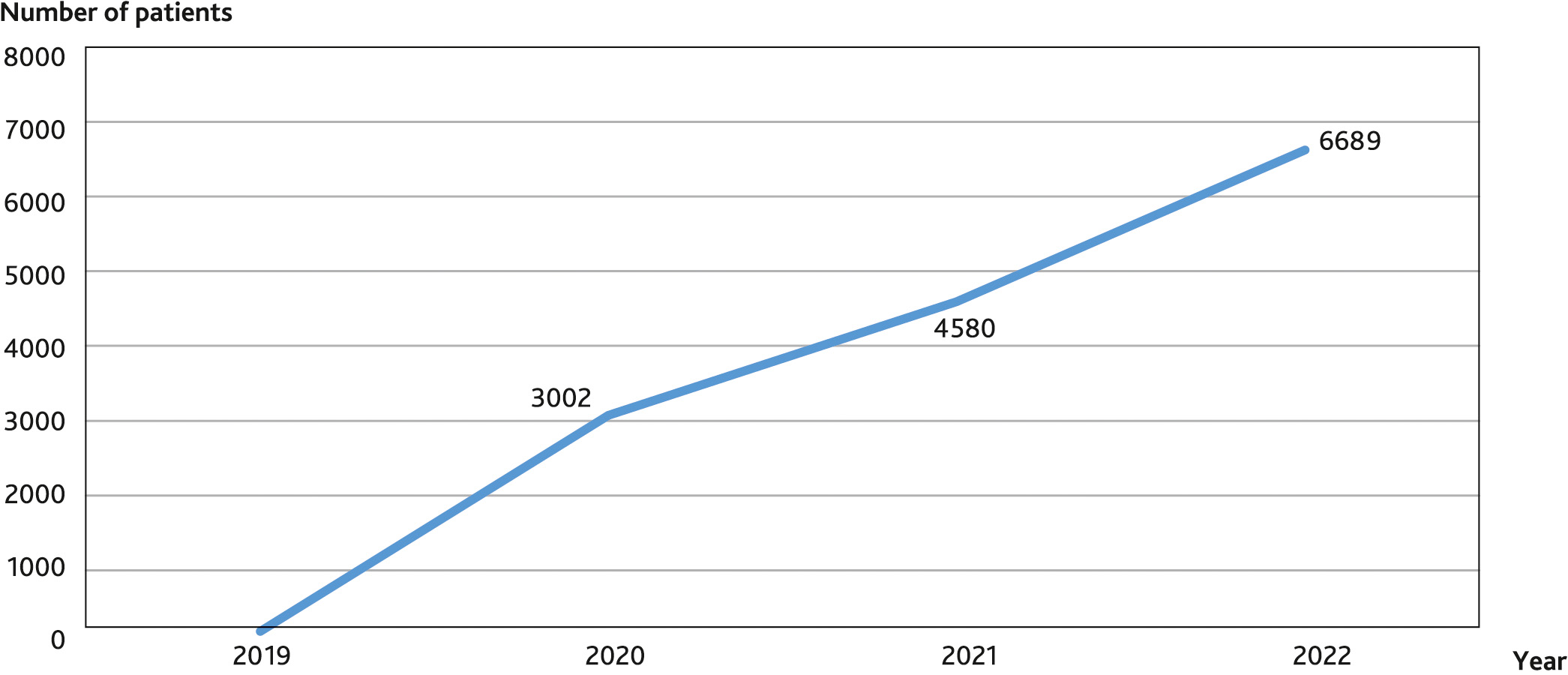
Fig. 2. Dynamics of enrollment of patients in the study
An interim analysis of the data showed that the probability of an unfavorable outcome can be assessed using factors such as the surgery severity level and the initial physical status, but its prognostic value for determining the risk of mortality is clearly insufficient, and its ability in assessing the risk of postoperative complications are even less [30].
As the analysis of 2022 showed, modern prognostic tools (ASA [American Society of Anesthesiologists] scale, the SORT scale [Surgical Outcome Risk Tool], the SRS scale [Surgical Risk Scale], the POSPOM scale [Preoperative Score to Predict Postoperative Mortality], the NZRISK scale [New Zealand RISK] and the SMPM scale [Surgical Mortality Probability Model]) have good prognostic value in assessing the risk of 30-day mortality after elective abdominal surgery. The ASA scale as the only assessment tool cannot be used to predict mortality and postoperative complications after abdominal surgery, the best results were found for the NZRISK and POSPOM scales, however, they do not accurately identify high-risk patients. Preliminary results, as well as foreign experience, allow us to conclude that the development of a national risk assessment tool based on the study of the contribution of concomitant diseases, age and severity of surgical intervention is a promising, urgent and extremely important medical and social task [31].
For the first time in Russian Federation, a multicenter study is planned and is being conducted to assess the risk factors for an unfavorable outcome in abdominal surgery. This study will allow to determine the role of concomitant diseases in the development of postoperative complications and mortality, as well as to create a national model for assessing perioperative risk.
Funding source. This study was not supported by any external sources of funding.
Disclosure. I.B. Zabolotskikh is the First Vice-President of the all-Russian public organization “Federation of anesthesiologists and reanimatologists”; A.I. Gritsan is the Vice-President of the all-Russian public organization “Federation of anesthesiologists and reanimatologists”; A.N. Kuzovlev is the Deputy Director of Federal Research and Clinical Center of Intensive Care Medicine and Rehabilitology and K.M. Lebedinskii is the President of the all-Russian public organization “Federation of anesthesiologists and reanimatologists”. Other authors declare that they have no competing interests.
Author contribution. All authors according to the ICMJE criteria participated in the development of the concept of the article, obtaining and analyzing factual data, writing and editing the text of the article, checking and approving the text of the article.
Registration of the study. The study was registered in the international database https://clinicaltrials.gov under the auspices of the All-Russian Public Organization “Federation of Anesthesiologists and Reanimatologists” (principal investigator I.B. Zabolotskikh), study number NCT03945968.