Against the background of the development of multiple protection neuroimmunoendocrine responses, a systemic inflammatory response syndrome is formed when a critical illness occurs. The trigger mechanism for the development of systemic inflammatory response syndrome in critical illness is an increase of the level of damage-associated molecular patterns and/or an increase of the level of pathogen-associated molecular patterns [1, 2]. The neuroimmunoendocrine response in critical illness is shaped by integrating influx of information from the vagus nerve, peripheral cytokine interactions with receptors in the area of circumventricular organs, brain vessels and local cytokine formation within the central nervous system [3–5]. The circumventricular organs are specialized structures located along the midline of the brain along ventricles III and IV [3, 6, 7]. These brain structures have lack of the blood-brain barrier and they are “windows to the blood circulation system” allowing molecules such as proteins, peptide hormones, cytokines, and lipopolysaccharides to penetrate relatively freely into brain tissue. Thus, neurons and glial cells (microglia and astrocytes) located in the circumventricular organs have access to macromolecules. Some of the organs surrounding the ventricles of the brain have neuronal contacts with groups of hypothalamic nuclei that regulate homeostasis [3].
Regardless of the reasons of hospitalization in intensive care units, the stress response of the neuroendocrine system proceeds within the framework of a biphasic model — acute and chronic phases [8].
The neuroendocrine system is able in the acute phase of the critical illness to turn the vector of the body's energy expenditures to such processes as gluconeogenesis, lipolysis, proteolysis, in order to overcome the prohibitive needs of the body. At the same time, anabolic processes are shifted to the recovery period.
70 % of patients in the acute phase of critical illness of various etiologies observed abnormal metabolism of thyroid hormones (total and free triiodothyronine (T3) and thyroxine (T4)), increasing reverse T3 and inhibition of thyrotropin (TSH). That is, TSH and T4 levels remain within reference values, while T3 levels decrease already in the first hours of critical illness [8]. This is associated with the ability of interleukin 1β, interleukin-6 and tumor necrosis factor α to mimic acute stress-induced changes in the pituitary gland; with increased levels of reversible T3; decreased peripheral conversion of T4 to T3; changes in the levels of proteins binding thyroid hormones; changes in thyroid receptor sensitivity. This phenomenon is called euthyroid pathology syndrome [9].
In case of non-recovery of vital functions within several days and the development of multiple organ failure syndrome there is a transition from the acute phase to the chronic phase of critical illness [8]. The transition from one phase to another is based on: increasing systemic level of proinflammatory cytokines; tissue hypoxia; dysoxia; hypoxic-ischemic brain damage; pathological permeability of capillary endothelial cells that form the blood-brain barrier. Therefore, there is an excessive penetration into the brain of proinflammatory mediators and other neurotoxic molecules, such as urea [10]. Circulating proinflammatory cytokines, such as interleukin 1, interleukin-6 and tumor necrosis factor α, penetrate through the blood-brain barrier damaging the nuclei of the limbic, hypothalamic and noradrenergic systems, leading to impaired neuroendocrine response [11].
Previously, the data obtained on the neuroendocrine system in the acute period of critical illness in the form of catabolism against the background of sepsis and septic shock were extrapolated without changes, to the chronic phase of critical illness. Currently, endocrine parameters monitoring data are becoming more available, and they are very different from those observed in the first few hours or days after the onset of critical illness [12].
In the chronic phase of critical illness, there is a decrease in thyroid hormone levels, and a lack of TSH response, manifested by a low-normal or reduced plasma TSH concentration. These changes in the pituitary-thyroid system are associated with high disease severity and an increased risk of death [13].
Whether changes in levels of TSH, FT3 and FT4 in the chronic phase of critical illness can be considered as an adaptation mechanism, or it is necessary to consider these changes as neuroendocrine dysfunction or depletion of the neuroendocrine system is an open question to date.
Treatment of critical illness and multiple organ failure syndrome due to different causes is the main task of modern intensive care. Various methods of treatment are used in intensive care, including methods characterized by high aggressiveness. Extracorporeal membrane oxygenation (ECMO) is a temporary highly aggressive method of life support in critical illness, primarily used in the development of severe respiratory and/or heart failure. ECMO is not an independent therapeutic measure, but only an organ replacement measure, giving time to maintain adequate blood circulation, oxygenation of organs and tissues, and conducting pathogenetically sound therapy aimed to restore the damaged organ or system [14]. At the same time, ECMO has an independent additional negative impact on the functioning of organs and systems through the development of a cascade of inflammatory reactions (coagulopathy, increased levels of cytokines, complement system, endothelial dysfunction) when the blood comes in contact with the artificial circulatory circuit [15].
The severity of the patient's condition, requiring, in addition to pharmacotherapy and mechanical ventilation, highly aggressive ECMO treatment and, the observed changes in the pituitary-thyroid system (reduced levels of T4 and T3, low-normal or reduced levels of TSH), can be considered as irreversible changes in the neuroendocrine system.
To date, there are no data on changes in the pituitary-thyroid system in adult patients during ECMO.
Objectives. The purpose of our study was to analyze the changes in plasma levels of TSH, free T4, free T3 during ECMO, at ECMO weaning/death on ECMO.
Forty-seven patients requiring ECMO were included in a prospective observational single-center study. Connection to ECMO occurred both at the stage of the primary hospital, with subsequent transportation to the ECMO center, and in the intensive care unit at the ECMO center, where their further treatment was performed.
Inclusion criteria: patients older than 18 years for ECMO. Exclusion criteria: age less than 18 years, pregnancy, brain death, use of synthetic glucocorticoids. The patients were monitored during ECMO in the intensive care unit of State Scientific Center of A.I. Burnazyan Federal Medical Biophysical Center of the Federal Medical and Biological Agency.
The follow-up period of the patients started from the moment of ECMO connection and was kept until weaning from ECMO/death on ECMO. Demographic characteristics were represented by descriptive statistics. The anamnesis was collected according to the medical records, and the moment of connection and duration of ventilatory ventilation before ECMO were recorded. Clinical and neurological examination included assessment of the patient's condition according to the following scales: The Acute Physiology and Chronic Health Evaluation (APACHE II), the Sequential Organ Failure Assessment (SOFA).
Criteria for initiation of veno-venous ECMO: respiratory index < 50 mmHg for > 3 hours; respiratory index < 80 mmHg for > 6 hours; arterial blood pH < 7.25 with hypercapnia > 60 mmHg for > 6 hours amid increased respiratory rate up to 35 times per minute [16–18].
In the heart failure (decrease of cardiac index < 2 l/min/m2 despite the use and ineffectiveness of vasopressor/inotropic doses) [19]. In order to maintain blood circulation in refractory cardiogenic shock, veno-arterial ECMO (VA ECMO) was used [20, 21].
In order to maintain blood circulation in refractory cardiogenic shock (systolic blood pressure below 90 mmHg and signs of severe organ hypoperfusion — disorders of mental functioning, water diuresis below 30 ml/hr, cold feet or hands, pulmonary edema, use and ineffectiveness of inotropes, vasodilators, intra-aortic balloon counterpulsation) we used veno-arterial ECMO [19, 20].
When arterial blood saturation is above 95 %, carbon dioxide tension is less than 50 mmHg, oxygen fraction level is not more than 50 % and the performance of veno-arterial ECMO is close to the cardiac output of the patient, there was a transition to the basic algorithm of patient weaning from ECMO [21].
Weaning from veno-arterial ECMO was based on the following parameters: patient status assessment, doses of inotropic and vasopressor support, resolution of organ dysfunction, respiratory index > 100 mmHg [22].
Blood sampling was performed after connection to ECMO — day zero (D0), then the first, third, fifth, seventh days (D1, D3, D5, D7) and further on every second day until weaning/death on ECMO. The pituitary-thyroid system (TSH, free T3 (FT3) and free T4 (FT4)) was evaluated by immunochemiluminescent and immunochemical methods. The time of blood sampling for TSH, FT4, and FT3 on D0 depended on the time of connection to ECMO. Subsequent blood sampling from the central venous catheter was performed until 8:00 a.m.
As part of the study, it was decided to focus on the existing reference values of TSH, FT3 and FT4 in blood plasma. When statistically significant results were obtained, the identified differences were indicated within the limits of the reference values.
The following were monitored in ECMO patients: saturation, partial pressure of oxygen and carbon dioxide, plasma lactate level, serum or plasma electrolyte concentrations, acid-base balance. Blood sampling to monitor the above parameters was performed from 4 places: from the cannula; after the oxygenator; from the central venous catheter; and from the patient's arterial blood.
The ethical committee of the State Scientific Center of A.I. Burnazyan Federal Medical Biophysical Center of the Federal Medical and Biological Agency (protocol № 9 dated April 25, 2016) approved this study.
Statistical data processing was performed using IBM SPSS Statistics. Descriptive statistics methods (Me — median, Q1 — first quartile, and Q3 — third quartile) were used to evaluate the study groups. The reliability of the data was determined by nonparametric criteria. Intra-group correlation was assessed by Spearman rank correlation coefficient (rs). Significance of differences between two unrelated groups was assessed by Mann—Whitney test (U). Wilcoxon criterion (W) was used to estimate parameter change over time for two related samples. Diagnostic significance of identified intergroup differences was compared using ROC curves, with sensitivity and specificity calculations. The critical level of significance for null hypothesis testing was ≤ 0.05.
Forty-seven patients were included in the study (Table 1). Indications for ECMO were determined as acute respiratory failure without correction by artificial ventilation — 40 (85 %); acute cardiovascular failure refractory to any other therapy (circulatory failure) — 7 (15 %).
The cause of acute respiratory dysfunction: respiratory distress syndrome — 40 patients (85 %). Cause of cardiogenic shock: myocarditis — 3 patients (7 %); acute myocardial infarction — 2 patients (4 %); cardiac arrest — 2 patients (4 %).
Patients who died after weaning from ECMO or died on ECMO — 29/47 (62 %). Cause of death: septic shock, 22/29 (75.9 %); pulmonary embolism, 2/29 (6.9 %); cardiogenic shock, 3/29 (10.3 %); hemorrhagic complications — 1/29 (3.45 %); brain death, 1/29 (3.45 %).
“ECMO weaning” is a procedure in which the ECMO system is removed due to restoration of lung gas exchange and/or cardiac function. Subsequently, these patients could have survived or died at any stage after ECMO weaning. We have indicated the fatal outcome during ECMO as “death on ECMO”.
Table 1. General characteristics of patients who survived and non-survived on ECMO
| Parameters | Survivors, n = 18 (38.3 %) | Non-survivors, n = 29 (61.7 %) | p |
|---|---|---|---|
| Age, age, Ме (Q1; Q3) | 40.5 (32; 46) | 50 (35; 60) | 0.039 |
| Sex, male/female | 29/19 | – | |
| BMI, kg/m2 | 27.6 (24.1; 28) | 26.4 (25.7; 30.7) | 0.56 |
| SOFA on day of weaning/death on ECMO | 8 (5; 10.5) | 12 (12; 16) | 0.001 |
| SOFA on day of ECMO connection | 9 (5.7; 12) | 12 (8.5; 13) | 0.18 |
| APACHE II on the day of ECMO | 19.5 (18.7; 22) | 24 (20; 31) | 0.01 |
| From the moment of connection to the mechanical ventilation before connecting to ECMO, day | 3 (1; 6) | 3 (1; 4) | 0.5 |
| Intensive care unit, day | 17 (14.2; 25.2) | 15 (5.5; 19.5) | 0.034 |
| Duration of ECMO, day | 7.5 (5.7; 10) | 8 (5; 15.5) | 0.594 |
| Duration of the targeted temperature management, 24 hours | 2 (2; 3) | 3 (3; 9) | 0.049 |
| Level of lactate in arterial blood on the day of connection to ECMO (mmol/l) | 2.8 (2.1; 4.4) | 3 (2.2; 4.6) | 0.9 |
| Arterial blood lactate level at day of weaning/death on ECMO (mmol/l) | 0.9 (0.6; 1.2) | 3.8 (2.1; 7) | 0.001 |
| Veno-venous ECMO | 16 | 24 | – |
| Veno-arterial ECMO | 2 | 5 | – |
| Diseases that led to the development of a critical condition and transfer to ECMO: | |||
| pneumonia | 16 | 24 | – |
| myocarditis | 1 | – | |
| cardiac arrest complicating myocardial infarction | 2 | 4 | – |
Before connection to BB ECMO, the oxygenation index against the background of 100 % oxygen in the inhaled gas mixture was less than 0.8. During veno-venous ECMO, we achieved the following target values of the arterial blood gas composition of the patient: oxygen tension greater than 60 mmHg; carbon dioxide tension = 45 mmHg; saturation = greater than 82; SpVO2 greater than 65 %.
The patient's baseline severity score (APACHE II) was higher in non-survivors. The age of patients who non-survived was higher than that of patients who survived. The duration of mechanical ventilation before ECMO was connected did not differ between the survivor and non-survivor groups. On the day of weaning/death on ECMO, the SOFA score was higher in non-survives. A longer stay in the intensive care unit was observed in patients with a favorable outcome.
There was no statistically significant difference between the patients who survived and those who died depending on the duration of ventilation before ECMO initiation.
Arterial blood lactate levels on the day of ECMO connection were not statistically significantly different in the groups of surviving and non-survivor patients. Total lactate level on the day of ECMO connection was 2.9 (2.2–4.4) mmol/l. On the day of weaning/death on ECMO, the lactate level was higher in non-survives patients.
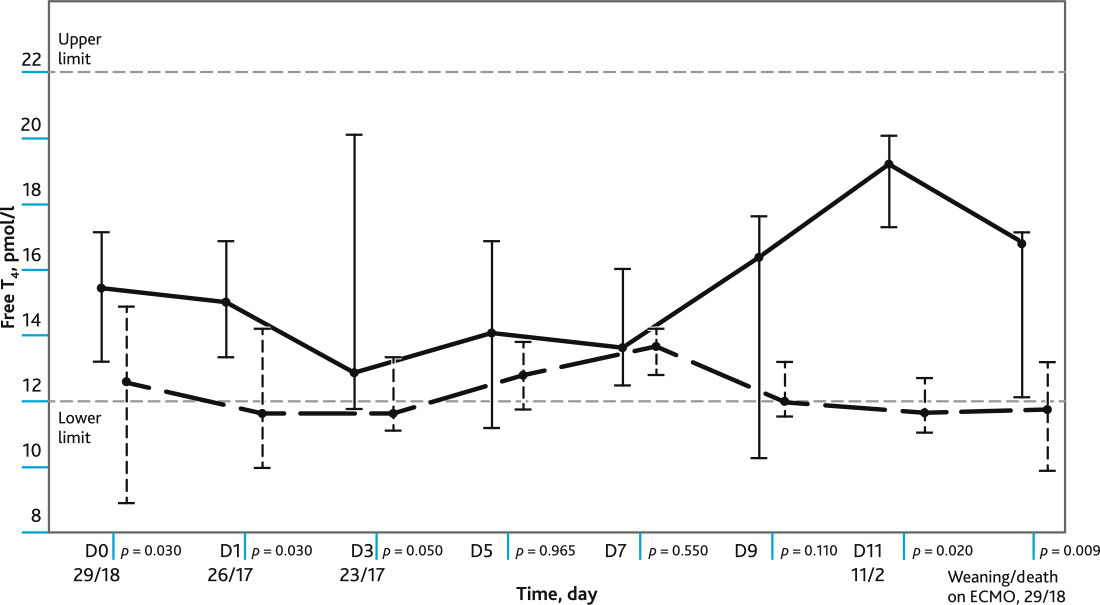
A comparative analysis of the plasma levels of FT4 at different time points in the groups of surviving and non-survives patients (Fig. 1). Plasma FT4 levels were lower in non-survives patients at D0, D1, D3, D11 and at the last day of follow-up. The reference values of FT4 level (12–22 pmol/l).

Fig. 1. The figure shows: the level of free T4 in dynamics in the groups of survivors and non-survived patients during ECMO; values of Me, Q1; Q3; reference values of the level of free T4. Marked day (D) of observation with a significance level (p) and number of non-survived/surviving patients. Solid line — survivors, dotted line — deceased
A comparative analysis of the plasma levels of FT3 at different time intervals in the groups of surviving and non-survives patients (Me; Q1; Q3) was performed (Fig. 2). Plasma levels of FT3 were lower in non-survivor patients at D5, D11 and the last day of follow-up. The reference values of FT3 level (3.1–6.8 pmol/l).
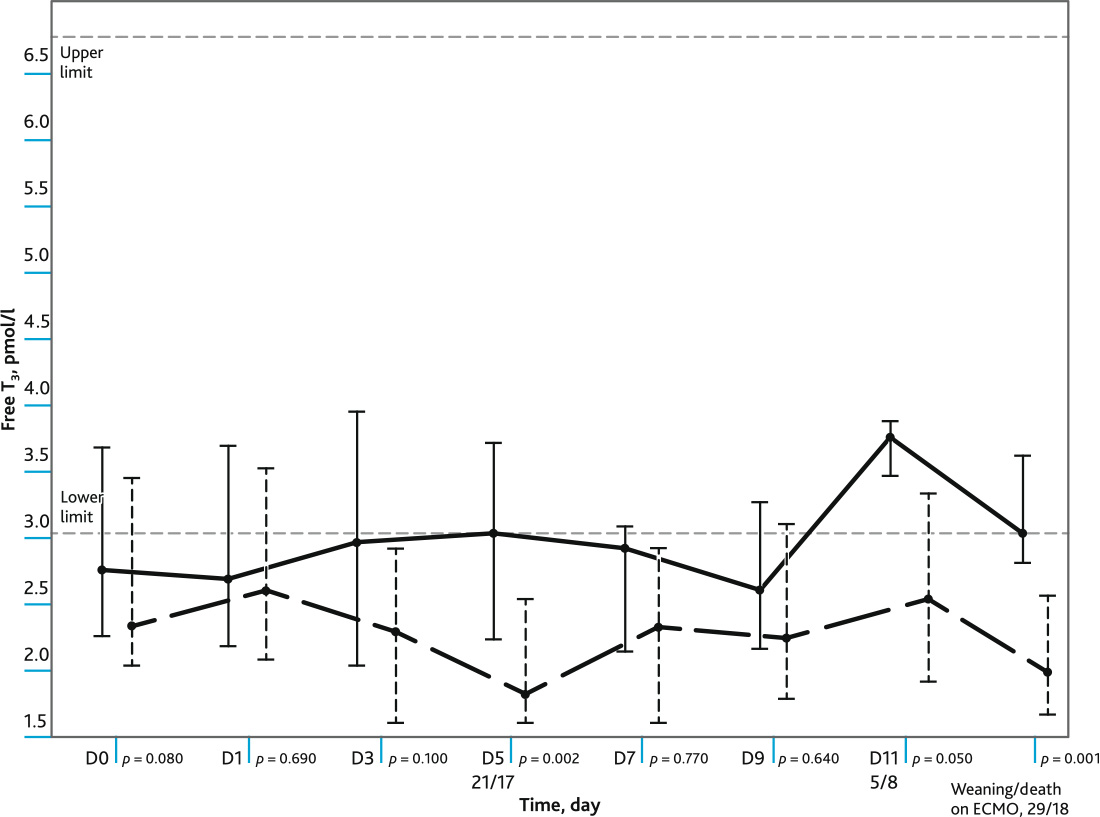
Fig. 2. The figure shows: the level of free T3 in dynamics in the groups of survivors and non-survived patients during ECMO; values of Me, Q1; Q3; reference values of the level of free T3. Marked day (D) of observation with a significance level (p), and number of non-survived/surviving patients. Solid line — survivors, dotted line — deceased
Differences in plasma TSH levels of non-survives and surviving patients during ECMO were observed only on the day of weaning/death on ECMO (Table 2). TSH normal values are 0.5 to 5.0 mIU/L.
Table 2. The table shows: TSH level in dynamics in the groups of survived and non-survived patients during ECMO; Me, Q1; Q3 values; Days (D) of observation with significance level (p) are marked. The number of surviving/non-survived patients (n) is shown in parentheses
| TSH, Day (D), n | Survivor, Me (Q1; Q3) | Non-survivor, Me (Q1; Q3) | p |
|---|---|---|---|
| D0 (18/29) | 0.9 (0.2; 1.9) | 0.7 (0.4; 2.1) | 0.93 |
| D1 (18/25) | 0.9 (0.06; 2.9) | 1 (0.4; 1.7) | 0.6 |
| D3 (17/25) | 1.57 (0.13; 2.9) | 0.51 (0.2; 2.6) | 0.93 |
| D5 (17/22) | 1.17 (0.4; 2.9) | 1.1 (0.16; 1.7) | 0.35 |
| D7 (12/18) | 1.18 (0.4; 2.8) | 1.8 (0.3; 3.1) | 0.47 |
| D9 (4/13) | 0.4 (0.2; 2.3) | 1.7 (0.3; 3) | 0.49 |
| D11 (1/11) | 2.5 (2.5) | 1.6 (0.1; 5.5) | 0.66 |
| Day of weaning/death on ECMO (18/29) | 1.2 (0.6; 2.49) | 0.35 (0.08; 1.23) | 0.01 |
A comparative analysis of plasma levels of TSH, FT3, and FT4 in the untreated and dopamine-treated patient groups (38/9) on the day of connection (D0) to ECMO and the last day of follow-up was performed. No statistically significant difference was found between patients without and with dopamine treatment (Table 3).
Table 3. Analysis of differences between patients with/without dopamine treatment at moment of ECMO connection initiation activation and on the day of weaning/death on ECMO, (Me, Q1; Q3)
| Day, (n) | Without dopamine treatment, n = 38 (81 %) | Dopamine, n = 9 (19 %) | p |
|---|---|---|---|
| D0 | |||
| TSH | 0.89 (0.51; 2.55) | 1.66 (0.26; 1.7) | 0.8 |
| FТ4 | 15.1 (11.5; 17.1) | 13.6 (9.3; 15.6) | 0.5 |
| FТ3 | 2.64 (2.1; 3.6) | 2.6 (2; 3.5) | 0.7 |
| Day of weaning/death on ECMO | |||
| TSH | 0.57 (0.17; 3.12) | 1.14 (0.2; 1.93) | 0.8 |
| FТ4 | 12.4 (11.6; 15.9) | 12.8 (9.2; 16.8) | 0.9 |
| FТ3 | 2.1 (1.9; 3.2) | 2.6 (1.8; 4.01) | 0.3 |
There was a negative correlation of arterial blood lactate levels with FT3 (rs = −0.519, p = 0.001); TSH (rs = −0.529, p = 0.001); FT4 (rs = −0.344; p = 0.035) on weaning/death on ECMO.
A statistically significant negative correlation was found on the day of weaning/death on ECMO of the SOFA scale score with TSH level (rs = −0.42, p = 0.003); FT4 (rs = −0.35, p = 0.015); FT3 (rs = −0.37, p = 0.01).
ROC curve analysis showed that low plasma levels of FT3 in patients on the day of weaning/death on ECMO demonstrated a sensitivity of 77 % and specificity of 93 % for adverse outcome (Fig. 3).
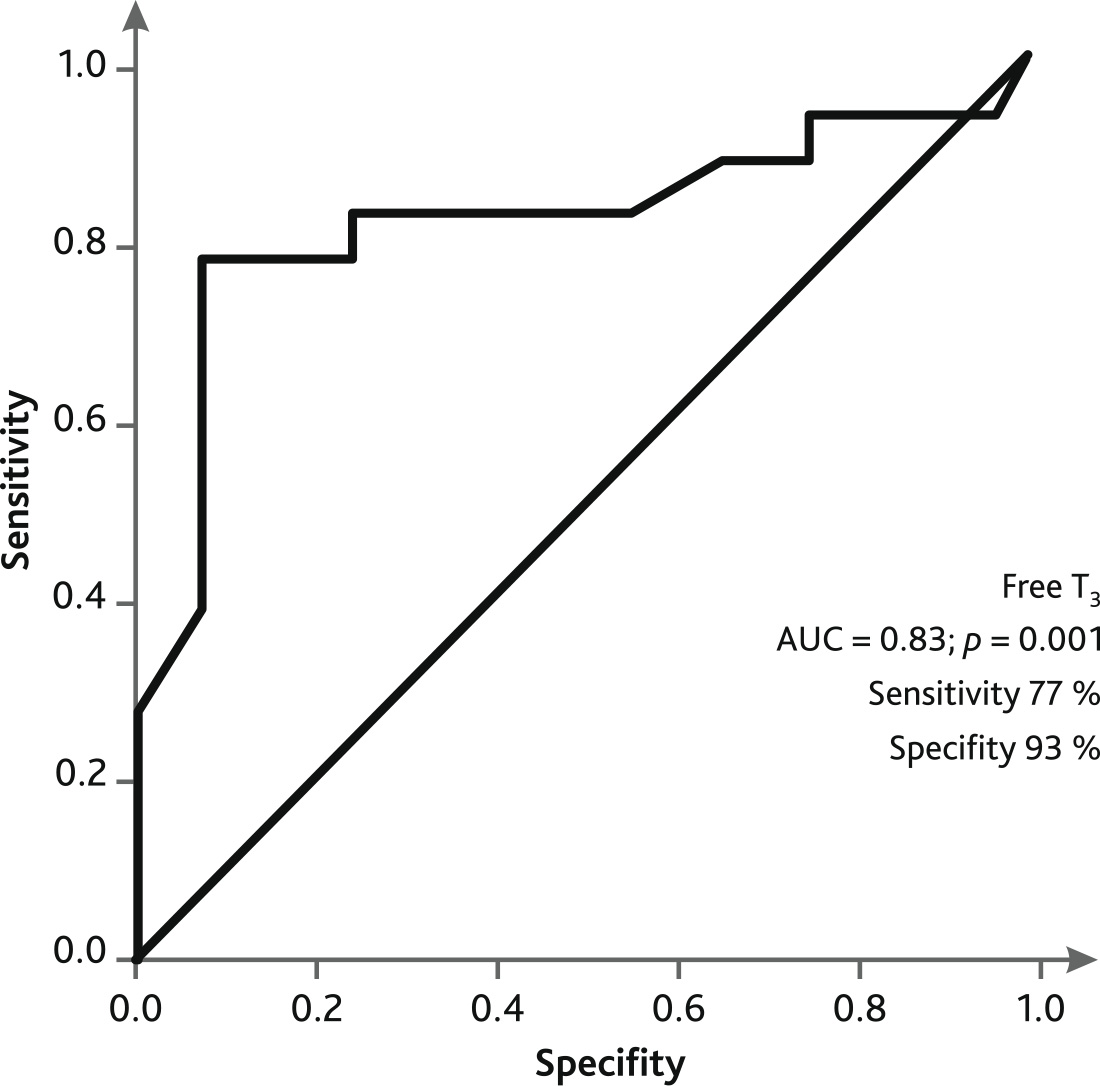
Fig. 3. ROC curve of predicting an unfavorable outcome on the day of excommunication/death on ECMO
ROC curve analysis showed that low plasma FT4 levels in patients on the day of weaning/death on ECMO demonstrated a sensitivity of 67 % and specificity of 89 % for adverse outcome (Fig. 4).
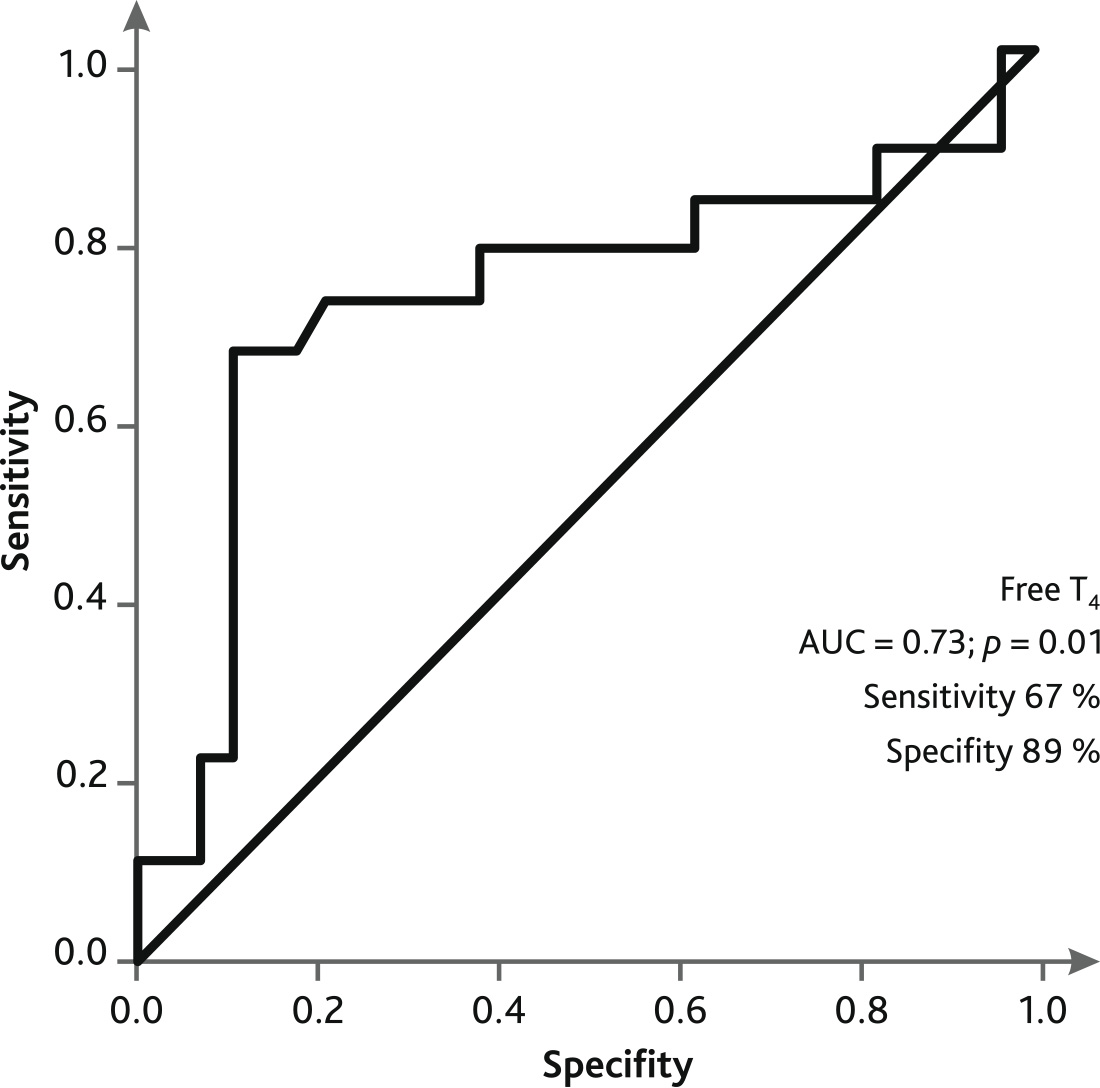
Fig. 4. ROC curve of predicting an unfavorable outcome on the day of weaning/death on ECMO
ROC curve analysis showed that low plasma TSH levels in patients on day of weaning/death on ECMO demonstrated sensitivity of 83 % and specificity of 69 % for adverse outcome on ECMO (Fig. 5).
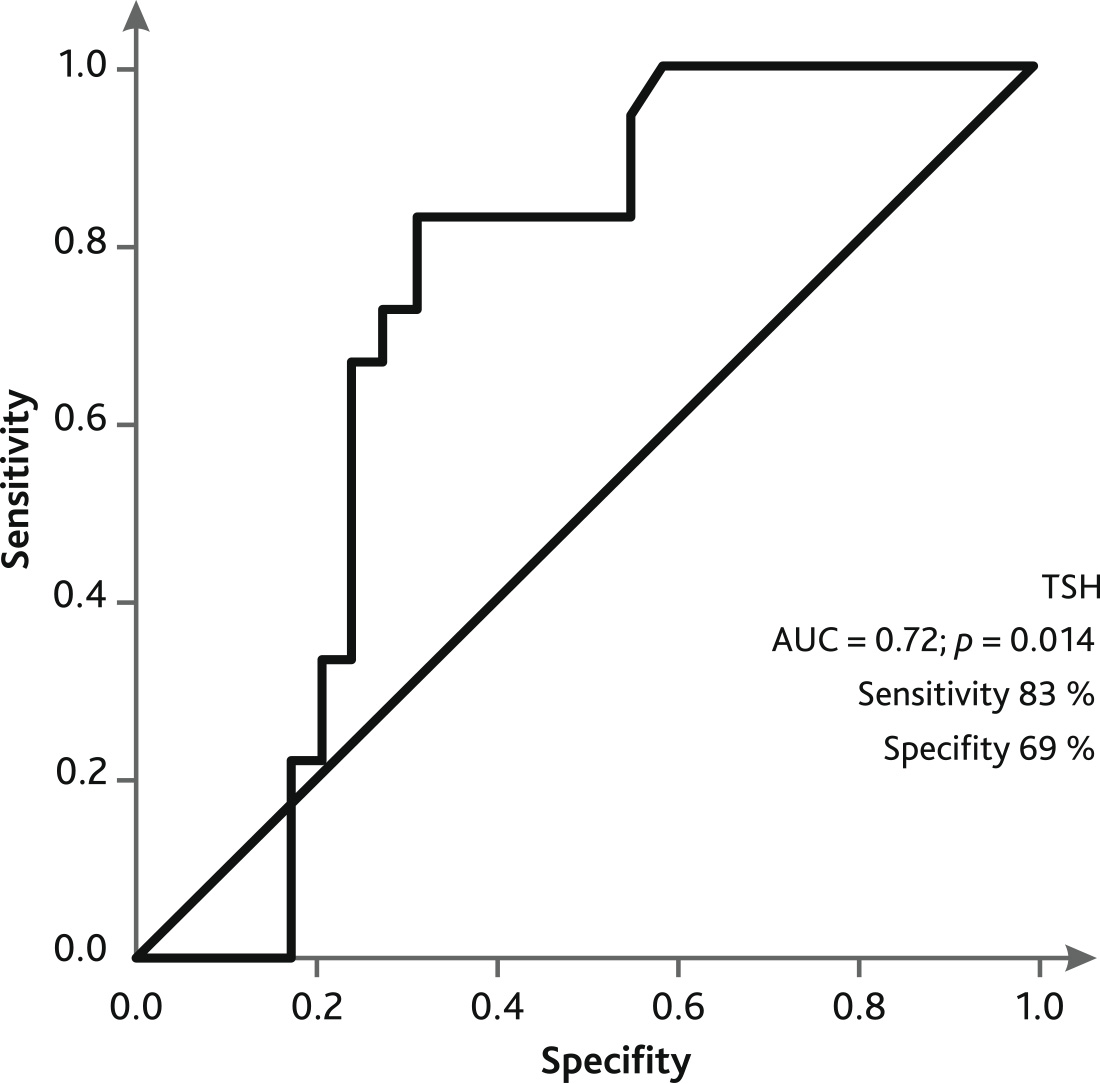
Fig. 5. ROC curve of predicting an unfavorable outcome on the day of weaning/death on ECMO
The biological effect of thyroid hormones depends on the coordinated functioning and interaction of all components of the hypothalamic — pituitary — thyroid-target tissue system [9]. Thyrolyberine, secreted by hypothalamic neurons through the pituitary portal system, promotes the synthesis and release of TSH into the bloodstream. Both TSH and thyroleiberin secretion are regulated by a negative feedback mechanism from T4 and T3 secreted by the thyroid gland. TSH release is also corrected by other hormones, including glucocorticoids and somatotropic hormone; it is suppressed by cytokines in the pituitary and hypothalamus [23].
Severe physical stressors are determinants of TSH secretion regardless of thyroid hormone levels and circadian rhythms [24]. Thus, in the euthyroid syndrome developing in the acute phase of critical illness (from several minutes to days) — low T3 content does not cause a compensatory increase in TSH secretion. The low concentration of T3 is due to a change in the vector of energy expenditures aimed at overcoming the prohibitive needs of the organism in the acute phase of critical illness in the form of gluconeogenesis, lipolysis and proteolysis [25, 26]. The above-mentioned changes develop when type 1 deiodinase activity decreases or when type 3 deiodinase is activated in the peripheral tissues of the body [27, 28].
Continued dependence of a patient on life support devices for several days or more is considered by Greet Van den Berghe et al. as formation of subacute phase of critical illness, and more than 14–21 days — as chronic phase of critical illness [29]. The above three-phase model of critical illness (acute, subacute, chronic phase) is characterized by fluctuations of cortisol and ACTH levels in blood plasma. Thus, Greet Van den Berghe suggests that damage to the patient's neuroendocrine system underlies the subacute and chronic phases of critical illness. Taking this model as the basis, based on the data obtained in our study about changes in levels of TSH and thyroid hormones during ECMO, we observe the transition of the acute phase into the subacute phase of critical illness. In addition, the moment of initiation of the patient's ECMO can be considered as the onset of subacute phase of critical illness.
It is the transition to the subacute phase is manifested by low-normal or low secretion of TSH, with decreased T4 and more suppressed T3, despite the achievement of full nutritional support. With these changes in the pituitary-thyroid system, the development of central hypothyroidism is most likely [30]. The development of central hypothyroidism in critical illness patients can be caused by damaging effects of: peripheral and local cytokines; hypoxia on hypothalamus, pituitary gland; long-term use of drugs for anesthesia and sedation, and, as a result, low amplitude of TSH level during a day, reduced thyroid activity [5, 31, 32]. In subacute phase of critical illness, low T3 concentration is not an adaptive mechanism of the body, since the value of acute T3 decrease is associated with the severity of the disease and the risk of lethal outcome [33, 34].
At autopsy of the brains of patients who died in subacute and chronic phase of critical illness, the expression of thyrotropin-releasing hormone (TRH) gene in paraventricular nuclei was lower than that in those who died in acute phase of critical illness, for example from acute trauma [35]. In addition, a positive relationship was observed between matrix ribonucleic acid expression of TRH and plasma concentrations of TSH and T3. At the same time, in surviving patients in the chronic phase of critical illness, an increase in the level of TSH is considered to be a good prognostic sign. Another explanation for the decrease in the amplitude of TSH secretion is an increase in type 2 deiodinase activity in the hypothalamus and pituitary gland and, as a consequence, transformation of T4 into active T3. This local increase in thyroid hormones in the hypothalamus and pituitary gland is perceived by the central nervous system as an excessive secretion of T4 and T3, which in turn suppresses both TRH and TSH activity. Therefore, the synthesis and secretion of thyroid hormones decreases [36, 37]. That is, the central nervous system suppression of thyroid activity is caused by damage of TRH nuclei and increased intracellular levels of thyroid hormones (T3 and T4) in the hypothalamus.
Under such conditions peripheral tissues adapt to thyroid hormone deficiency by increasing the number of intracellular thyroid hormone transporters, local activation of thyroid hormone (increase of type 2 deiodinase) and expression of active receptor isoform genes [37, 38, 39]. The experimental work of S.-F. Ma et al. showed the protective role of type 2 deiodinase activation in lungs in the development of sepsis and acute lung injury [40].
In critical illness, the use of exogenous dopamine and dexamethasone can provoke the development of central hypothyroidism [42, 43]. In our study, dexamethasone was not used according to exclusion criteria. No differences in TSH and thyroid hormone levels were found between the groups receiving and not receiving dopamine. This may be due to the short duration of dopamine administration and/or the limited number of patients who received it in the study. According to the results of this study, patients who died on ECMO and those who died after weaning from ECMO had lower FT4 levels below the reference values compared with those who survived.
It is possible that long-term use of drugs used for anesthesia and sedation influences the degree of inhibition of thyroid hormones and TSH levels during critical illness and ECMO [3, 44].
On the day of connecting patients to ECMO there was a statistically significant difference in plasma T4 level (within the reference values) in the groups of subsequently survived and non-survivor patients. The FT4 level in the subsequently non-survivor patients was at D0 low-normal; in the survivors it was significantly higher. Similar results were obtained in earlier studies in patients without ECMO [45].
The patients who died subsequently on the day of connection to ECMO and at D1, D3 had severely depressed plasma levels of FT3 In the group of survived patients, on the day of ECMO connection, plasma FT3 content was below the reference values, subsequently from D5 FT3 level increased to low-normal values. Statistically significant differences between the groups of surviving and non-survivor patients were observed at D5, D11 and on the day of weaning/death on ECMO.
In the group that died on ECMO or in patients who died subsequently, plasma levels of TSH, FT3, and FT4 were negatively correlated with the SOFA score.
Throughout the follow-up, plasma TSH levels did not differ in the groups of patients who survived and died on ECMO or in patients who subsequently died. TSH levels in the non-survivor groups were statistically significantly lower than in the survivor group only in the last day of follow-up.
The observed decrease in plasma levels of TSH, FT4 and FT3 on the day of patient weaning from ECMO is an independent predictor of adverse outcome. According to R.P. Peeters et al. results, decreased levels of TSH and thyroid hormones are associated with probable adverse outcome in severe community-acquired pneumonia [46].
Changes observed in levels of thyroid hormones and TSH, in patients in the subacute phase of critical illness during ECMO, indicate depletion, or irreversible damage to such brain structures as the noradrenergic system, hypothalamus and limbic system [5], which in our study is confirmed by the observed negative correlation of low levels of TSH, FT4, FT3 with both high lactate levels and a high SOFA score. Lactate is a surrogate marker that is one of the significant indicators of generalized tissue hypoxia [47]. Low levels of TSH and thyroid hormones in plasma and their negative correlation with arterial blood lactate levels on the day of weaning/death on ECMO is a sign of damage to the nuclei (the central nervous system) regulating the behavior of the neuroendocrine system in the critical illness. Along with the above, we observed recovery of pituitary-thyroid system in surviving patients; that is, we can say that, during ECMO, increases in thyroid hormone and TSH levels in the dynamics are a sign of favorable outcome.
In the format of the study, etiopathogenetic heterogeneity of groups (BB ECMO, VA ECMO) was not significant, because the formation and course of critical illness are typical regardless of the causes that caused them [48]. At the same time, the method of connection to ECMO rather reflects the severity of the condition than the peculiarities of the effect of its connection variants on the pituitary-thyroid system.
Within the framework of the three-phase model of critical illness (acute, subacute and chronic phases), the patient's condition requiring connection to ECMO is considered as a subacute phase. This conclusion is based on the initiation of connection to ECMO (from the moment of development of critical illness until connection to ECMO); on fluctuations of FT3, FT4 and plasma TSH levels in the groups of survivors and non-survivors throughout the study; and on negative correlation of lactate levels with TSH, and with thyroid hormones in blood plasma.
Along with high arterial blood lactate levels and a high SOFA scale score, the degree to which FT3, FT4, and TSH levels decreased in patients in critical illness during ECMO correlated with survival prognosis.
Low plasma levels of TSH, FT3, and FT4 are independent predictors of adverse outcome at the time of weaning/death on ECMO.
Disclosure. The authors declare that they have no competing interests.
Author contribution. All authors according to the ICMJE criteria participated in the development of the concept of the article, obtaining and analyzing factual data, writing and editing the text of the article, checking and approving the text of the article.
Ethics approval. This study was approved by the local Ethical Committee of A.I. Burnasyan Federal Medical Biophysical Center FMBA (reference number: 9-25.04.2016)
Funding source. This study was not supported by any external sources of funding.