The new SARS-CoV-2 virus emerged in late 2019 and led to one of the most worldwide pandemics in modern history. About 20% of COVID-19 patients exhibit moderate to severe symptoms [1]. Severe COVID-19 manifests as acute respiratory distress syndrome (ARDS) and is caused by alveolar damage and decrease blood oxygenation [2]. The main pathogenic way of ARDS development is hyperactivation and dysregulation of the immune system, which is associated with elevated pro-inflammatory cytokines and chemokines producing, such as interleukin 6 (IL-6), interleukin 8 (IL-8), tumor necrosis factor-α (TNF-α), IP-10 (interferon-induced protein 10), monocyte chemoattractant protein-1 (MCP-1). Clinical reports show that mild and severe forms of disease result in changes in circulating leukocyte subsets [3]. The severe course of COVID-19 is caused mainly by the immune system hyperactivation [4]. Therefore, a new therapeutic strategy for COVID-19 is urgently needed to correct the immune response and reduce the level of pro-inflammatory cytokines. The targeting of the hyperactive systemic inflammatory response by mesenchymal stem cells (MSCs) infusion opens up a new therapeutic strategy for therapy of COVID-19-associated acute respiratory distress syndrome [5]. Numerous clinical studies prove that MSCs could substantially improve the outcomes of uncontrolled immune activation followed by tissue damage, such as acute lung injury caused by the H9N2 virus, ARDS, autoimmune diseases and graft-versus-host disease [6–12]. Thus, MSC infusion may be an attractive tool in the treatment of severe COVID-19 infection. One of the first pilot clinical trial of intravenous MSC infusion performed on seven patients with COVID-19 infected pneumonia (Beijing YouAn Hospital, Capital Medical University, China) demonstrated positive clinical outcomes without any visible side effects.
The unique ability of MSC to regulate both the immune response and tissue regeneration makes them a good therapeutic tool in the treatment of severe forms of COVID-19. Notably, a recent study showed that MSC didn’t express the angiotensin-converting enzyme 2 receptor (ACE2) and it prevented MSCs’ infection by SARS-CoV-2 virus [13].
The results of several clinical trials show that MSC due to various mechanisms are able to suppress the cytokine storm in patients with COVID-19 by regulating the balance of pro-inflammatory and anti-inflammatory cytokines [14]. In addition, the application of MSC provides the restoration of subpopulations of CD4+ and CD8+ T cells, which are depleted during COVID-19 [15]. Thus, the use of MSCs can provide the suppression of inflammation and the regulation of immune homeostasis in patients with severe COVID-19.
Objective. The objective of the study — to evaluate of the effect of mesenchymal stem cell (MSC) therapy on the course of severe forms of novel coronavirus infection, accompanied by acute respiratory distress syndrome and “cytokine storm”, caused by hyperactivation of the immune system, the duration of stay on ALV and patient survival rate.
A prospective single-center study included 39 patients with severe forms of coronavirus infection who underwent therapy at the Department of Anesthesiology and Intensive Care No. 2 (DAIC No. 2) of the State Institution “Minsk Scientific and Practical Center for Surgery, Transplantology and Hematology” during 2020–2021. The severity of the condition of these patients was due to hypoxia affected by coronavirus infection and developing complications. Patients were divided into 2 randomized groups: control group (n = 16) and main group (n = 23). The study was approved at the ethics committee of the State Institution “Minsk Scientific and Practical Center for Surgery, Transplantology and Hematology” (No. 5 dated June 12, 2020). All participants signed written consent for participation in the study or another document in accordance with the regulatory legal acts of the Republic of Belarus.
Inclusion criteria:
Exclusion criteria:
Upon admission to the DAIC No. 2 all patients were rated with help of Sequential Organ Failure Assessment (SOFA) [16], Acute Physiology and Chronic Health Evaluation (APACHE) II [17], Acute Respiratory Distress Syndrome (ARDS) [18], their physical parameters were taken into account including gender, age, body mass index (BMI) (Table 1).
Table 1. Main and control groups specifications
| Parameters | Main group | Control group | Index р |
|---|---|---|---|
| Age | 49.5 (34; 76) | 48.5 (37.75; 63.75) | 0.647 |
| Male sex, n (%) | 18 (78%) | 8 (50%) | – |
| BMI, kg/m2 | 28.5 (27.7; 31.95) | 30.0 (25.7; 33.1) | 0.484 |
| SOFA, grades | 6.4 (4; 10) | 7.5 (5; 8.25) | 0.943 |
| APACHE II, grades | 11 (9; 17) | 14 (11; 15.25) | 0.519 |
| ARDS, stage | 3 (2; 3) | 3 (2;3) | 0.859 |
| Type of respiratory support correlation (O2/ HFNC/ NIV/ ALV invasive), n (%) | 2 (8.7%) / 6 (26%) / 2 (8.7%) / 13 (56.5%) | 2 (12.5%) / 3 (18.75%) / 2 (12.5%) / 9 (56.25%) | – |
The relatively low median age of patients in both groups is probably due to the predominant hospitalization in the center and DAIC No. 2 patients up to 65 years old. Treatment of patients in the control and main groups of the study was carried out according to the current protocols for the diagnosis and treatment of a new coronavirus infection of the Ministry of Health of the Republic of Belarus, which significantly do not differ from the clinical recommendations for the treatment of coronavirus infection adopted in the Russian Federation and the countries of the European Union. At the time of inclusion in the study, patients in both groups received respiratory support in the form of oxygen therapy (O2), HFNC (high-flow oxygen therapy), NIV (non-invasive ventilation) and invasive mechanical ventilation. The ratio of types of respiratory support in the main group and the control group (O2, HFNC, NIV, invasive mechanical ventilation) was 2/6/2/13 and 2/3/2/9, respectively. Patients in the main group received additional MSC cell therapy.
A biomedical cellular product “Human mesenchymal cells, ТУ BY 100660677.001-2014, izm. 1” (registration number БК-7.3/7.003-2007 in the State Register of Medical Devices and Medical Equipment of the Republic of Belarus) was used for cell therapy of the main group's patients. It was produced by the State Institution “Minsk Scientific and Practical Center for Surgery, Transplantology and Hematology”. The use of a biomedical cellular product is regulated by the legal acts of the Republic of Belarus, approved by the decision of the ethical committee. MSCs were obtained from adipose tissue during multiorgan procurement procedure. Adipose tissue was treated with collagenase type I solution in proportion 1:1 and incubated for 60 min at 37.0 °С. Mononuclear cells were counted according to the standard method with acetic acid stained with methylene blue. Cell viability was conducted by exclusion of trypan blue. Cells were cultured at 37.0 °С, 5% CO2, 90% humidity. Morphological analysis of cultures was conducted on an inverted microscope (Micros, Austria and Nikon, Japan) with phase contrast. Isolated cells were classified as MSCs by flow cytometry analysis (positive for CD90, CD105, CD13, CD44, CD73, CD106, CD29 and negative for CD45, CD34, CD31, CD14, HLA-DR). Enumeration was carried out in the operation program FACSDiva. The cellular product was administered intravenously in 20–40 ml of a sterile 0.9% NaCl solution. MSC infusion was started immediately after delivery of the cellular product to the department and was carried out for 10–20 min with a 16G venous catheter at a rate of 2 ml/min.
Immunophenotyping of peripheral blood lymphocytes of patients with coronavirus infection (day 0, day 7) was carried out to investigate the profile of the immune system constitution during MSC transplantation. The following fluorochrome-labeled monoclonal antibodies were used: CD3-Pacific Blue (BeckmanCoulter, USA), CD8-Krome Orange (BeckmanCoulter, USA), CD25-APC (BeckmanCoulter, USA), CD4-APC-Cy7 (ExBio, Czech Republic), CD38-FITC (BeckmanCoulter, USA), CD 28 FITC (BeckmanCoulter, USA).
Plasma levels of pro-inflammatory cytokines of patients with COVID-19 before and after cell therapy (day 0, 3, 7) were determined by multiplex analysis on a Luminex 200 (Luminex, USA) using Human Cytokine/Chemokine Magnetic Bead Panel HCYTOMAG-60K (EMD Millipore Corporation, Germany) as part of a commercial test system consisting of 6 analytes: IL-6, IL-8, IL-10, TNF-α, MCP-1, IP-10.
Statistical analysis was performed by non-parametric methods to compare two dependent groups for one quantitative variables using the Wilcoxon Matched Pairs Test, to compare two independent groups for one quantitative variable using the Mann—Whitney U-test. The distribution parameters of quantitative variables were presented as a median with 25% and 75% (Q1; Q3) quartiles. P values < 0.05 indicated statistical significance. Statistical analysis was performed in SPSS Statistic 26.0 and Statsoft Statistica 10.0 software.
The effectiveness of the MSC therapy in the complex treatment of severe forms of coronavirus infection accompanied by the development of ARDS was evaluated based on the dynamics analysis of the following laboratory parameters: C-reactive protein (CRP), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), procalcitonin (PCT), D-dimers (DD) and plasma levels of cytokines (IL-6, IL-8, IL-10, TNF-α, MCP-1, IP-10). The comparison of disease’s course and outcomes in patients of the main and control groups (survival rate, duration of stay in the DAIC No. 2, duration of stay on mechanical ventilation) was also the basis for the analysis.
All patients from the main group (n = 23) received MSC cell therapy in the amount of 172.4 (95.5; 302.9) × 106 cells, which is equivalent to a dose of 1.93 (0.96; 2.87) × 106/kg of the patient's weight.
The laboratory parameters for 0, 3, 5, 7 days in the two groups of patients are presented in Table 2.
Table 2. Laboratory markers dynamic in main and control groups
| Parameter | Day | Main group, n = 23 Me (Q1; Q3) | Control group, n = 16 Me (Q1; Q3) | р value |
|---|---|---|---|---|
| LDH, U/l | 0 | 550 (417; 616.5) | 621 (362; 941.5) | 0.509 |
| 3 | 475 (358.5; 577.5) | 519 (383; 670.5) | 0.340 | |
| 5 | 388 (326.5; 584.5) | 560 (426.5; 718) | 0.017 | |
| 7 | 376 (292.5; 588) | 529 (381.5; 811.5) | 0.038 | |
| CRP, mg/L | 0 | 61 (32.5; 152) | 34.8 (14.9; 99.7) | 0.291 |
| 3 | 30 (11.8; 109.5) | 75.6 (23.7; 124) | 0.291 | |
| 5 | 20.9 (7.35; 56) | 101.6 (23.3; 165) | 0.363 | |
| 7 | 21.4 (7.95; 92.7) | 164 (39.5; 212.3) | 0.062 | |
| DD, μg/ml | 0 | 1512 (556; 3129) | 940 (403; 2468) | 0.371 |
| 3 | 1263 (628; 2787) | 535 (398; 1276) | 0.009 | |
| 5 | 1192 (684; 2468) | 794 (381; 1358) | 0.163 | |
| 7 | 997 (565; 2649) | 915 (463; 1328) | 0.428 | |
| AST, U/l | 0 | 43.7 (30.7; 115.3) | 66.5 (21; 130) | 0.953 |
| 3 | 42.4 (33.1; 94.4) | 66.8 (43.2; 198) | 0.211 | |
| 5 | 42 (30; 65.6) | 55.1 (37.5; 117) | 0.293 | |
| 7 | 40.6 (40.6; 60.7) | 53.1 (35.3; 193) | 0.108 | |
| ALT, U/l | 0 | 59.6 (33; 111.8) | 51.3 (45; 90.2) | 0.921 |
| 3 | 49.9 (33.3; 122.4) | 63.3 (51.1; 155.1) | 0.211 | |
| 5 | 49 (31.9; 96.6) | 53.9 (43.6; 194) | 0.293 | |
| 7 | 47.3 (27; 77.1) | 63.7 (32.6; 77.5) | 0.692 | |
| PCT, U/l | 0 | 0.52 (0.07; 1.87) | 0.28 (0.09; 1.49) | 0.706 |
| 3 | 0.3 (0.11; 1.91) | 0.25 (0.13; 0.72) | 0.858 | |
| 5 | 0.46 (0.07; 2.64) | 0.165 (0.06; 1.69) | 0.713 | |
| 7 | 0.41 (0.06; 3.46) | 0.305 (0.125; 4.18) | 0.843 | |
| ALT — alanine aminotransferase; AST — aspartate aminotransferase; CRP — C-reactive protein; DD — D-dimers; LDH — lactate dehydrogenase; PCT — procalcitonin. The Mann—Whitney U-test was applied to compare two independent groups. |
||||
Before the cell therapy, there were no statistical differences in both groups in laboratory parameters: LDH, CRP, DD, AST, ALT, PCT. An increase in these biochemical markers is characteristic of a severe course of COVID-19 and corresponds to other investigators reports [23].
A statistically significant decrease in the level of LDH in the main group (MSC cell therapy group), was recorded compared to the control group on day 5 (respectively 388 (326.5; 584.5) U/l and 560 (426.5; 718) U/l (p = 0.017, n = 39) and on day 7 (respectively 376 (292.5; 588) U/l and 529 (381.5; 811.5) U/l (p = 0.038 n = 39), which may be caused by decreasing tissue damage and increasing of regeneration. There were no significant differences in the concentration of hepatic enzymes in both groups. The values of these enzymes indirectly indicate the predominantly “pulmonary” origin of LDH as a universal marker of cell damage, and a statistically significant decrease in the activity of this enzyme on days 5 and 7 after MSCs therapy may indicate lung tissue regeneration.
CRP level in the main group was slightly higher than in the control group, although it did not reach statistical differences. There was a decrease in CRP level from 61 (32.5; 152) mg/l on day 0 to 21.4 (7.95; 92.7) mg/l on day 7 in the main group. In the control group there was an increase CRP concentration from 34.8 (14.9; 99.7) mg/l on day 0 to 164 (39.5; 212.3) mg/l on day 7. CRP is the main laboratory predictor of the severity of lung injury [24]. High CRP level correlates with the severity of the disease course and is the basis for starting anti-inflammatory therapy. The dynamics of CRP level shows a decrease of inflammation evidence in the main group’s patients with severe coronavirus, as the response to the MSC application compared with the control group on the 5th and 7th days, however, the difference was statistically insignificant. At the same time, during the study period, there was a clear trend in decreasing in the CRP concentration in the main group, unlike the dynamics of this indicator in the control group. The absence of significant differences in the parameters of the two study groups may be due to the rather long-term inflammation signs during therapy, a slow decrease in the concentration of inflammatory markers (the half-life of CRP is 19 hours), and the limitation of the study time to 7 days. The positive dynamics of CRP level in patients with severe coronavirus after the MSC application in our study shows a decrease in the inflammation signs and may be associated with the anti-inflammatory effect of MSC. These results coincide with the available clinical data of other researchers [3, 22].
We studied the plasma cytokines’ level, which play an important role in the development of acute inflammation and “cytokine storm” in the main group’s patients to evaluate the anti-inflammatory effect of MSC when used in the complex treatment of ARDS in coronavirus infection.
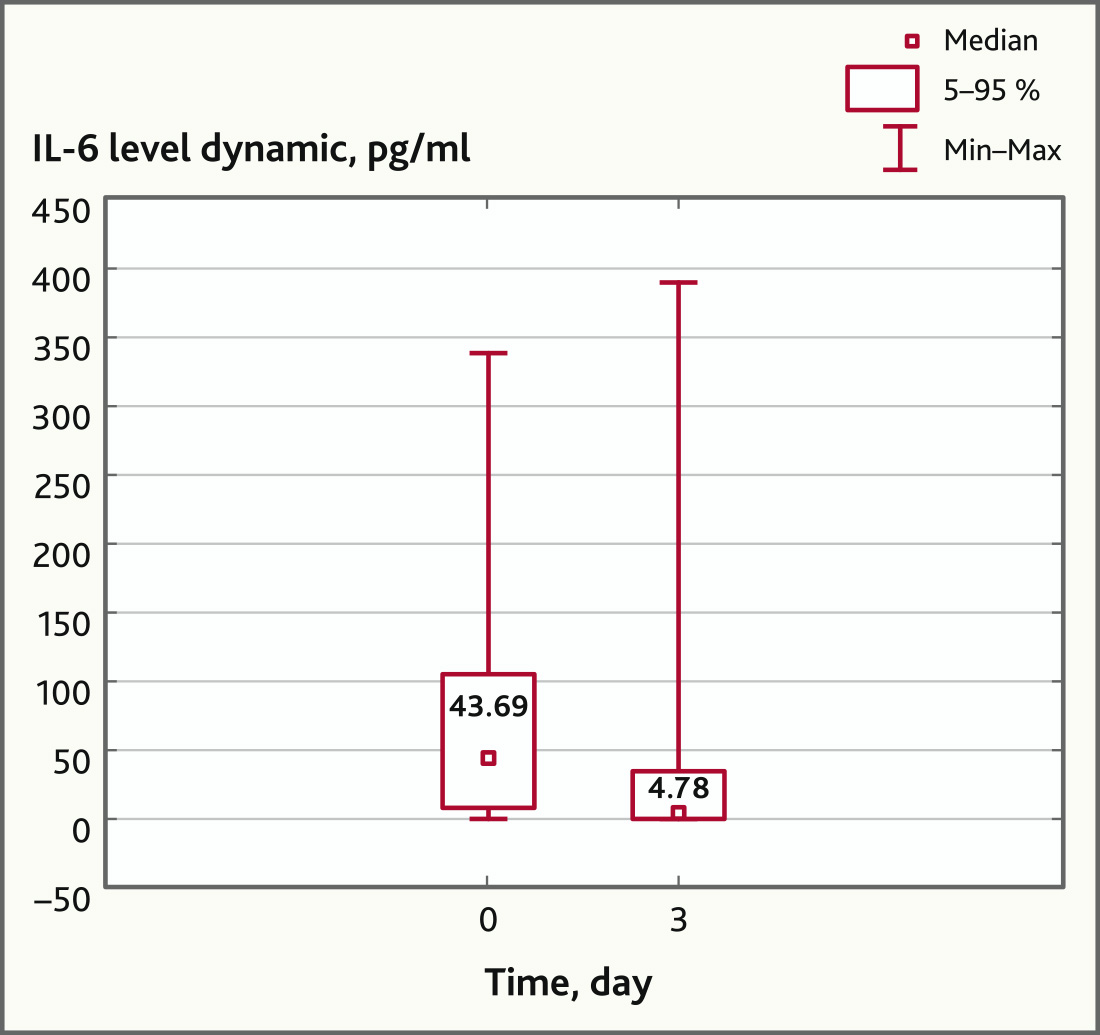
On the day 3 there was a statistically significant decrease in the IL-6 plasma level compared with the baseline (43.69 (1.74; 336.91) pg/ml and 4.78 (0; 388.09) pg/ml, respectively (p = 0.029, Wilcoxon Matched Pairs Test) (Fig. 1) in patients with ARDS treated with MSC, which is probably the result of the anti-inflammatory effect of MSC.

Fig. 1. IL-6 levels before and after MSC application (n = 27, p = 0.029)
Also on day 3, there has been a downward trend in the concentration of IL-8, which by day 7 acquired a statistically significant character: from 11.62 (0.98; 117.11) pg/ml before the MSC application to 8.96 (0; 83.62) pg/ml and 4.81 (0; 49.94) pg/ml on days 3 and 7, respectively (p = 0.049, Wilcoxon Matched Pairs Test) (Fig. 2).
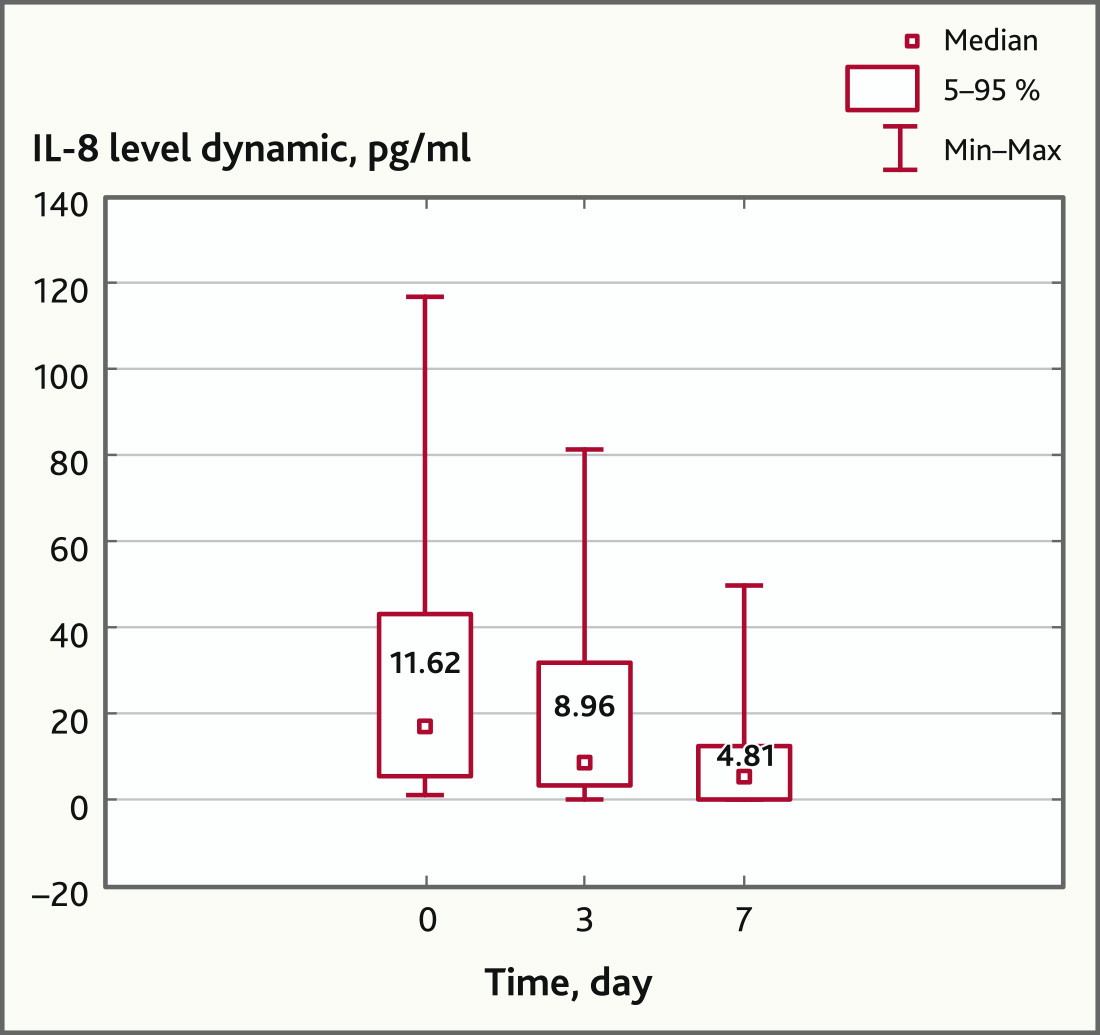
Fig. 2. IL-8 levels before and after MSC application (n = 17, p = 0.049)
In addition, the application of MSC contributed to a statistically significant decrease in the level of IL-10 and TNFα on day 7 (p = 0.019 and p = 0.006, respectively, Wilcoxon Matched Pairs Test). The dynamics of the level of these cytokines is shown in Fig. 3.
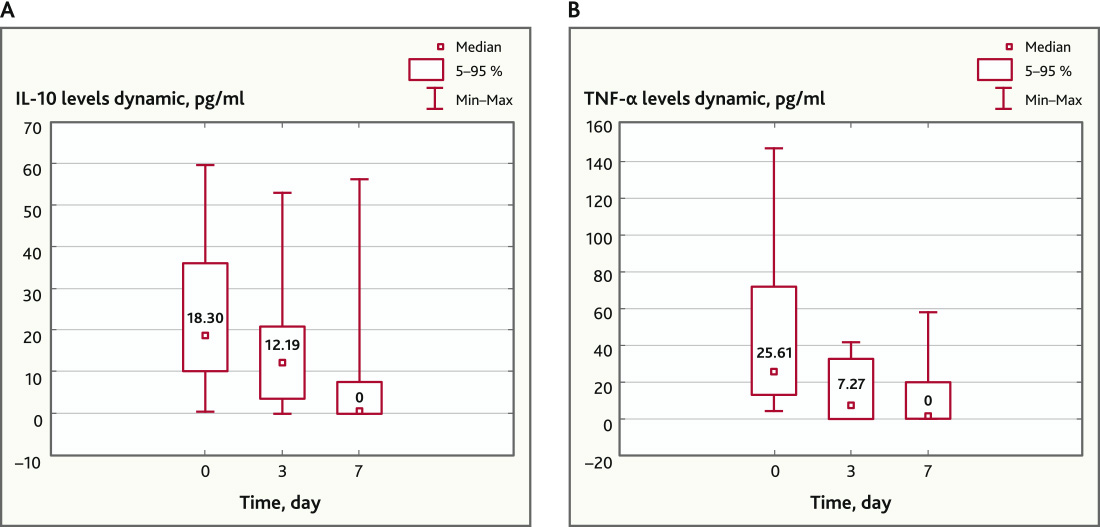
Fig. 3. IL-10 and TNFα levels before and after MSC application (n = 13). A — IL-10 levels dynamic, pg/ml; B — TNF-alpha levels dynamic, pg/ml
Besides, on day 3 after MSC application, a statistically significant decrease in the concentration of chemokines IP-10 (p = 0.017; n = 16) and MCP-1 (p = 0.019; n = 20) was recorded, however, by day 7, this regularity persisted only for IP-10 (p = 0.0004, n = 16, Wilcoxon Matched Pairs Test) (Fig. 4).
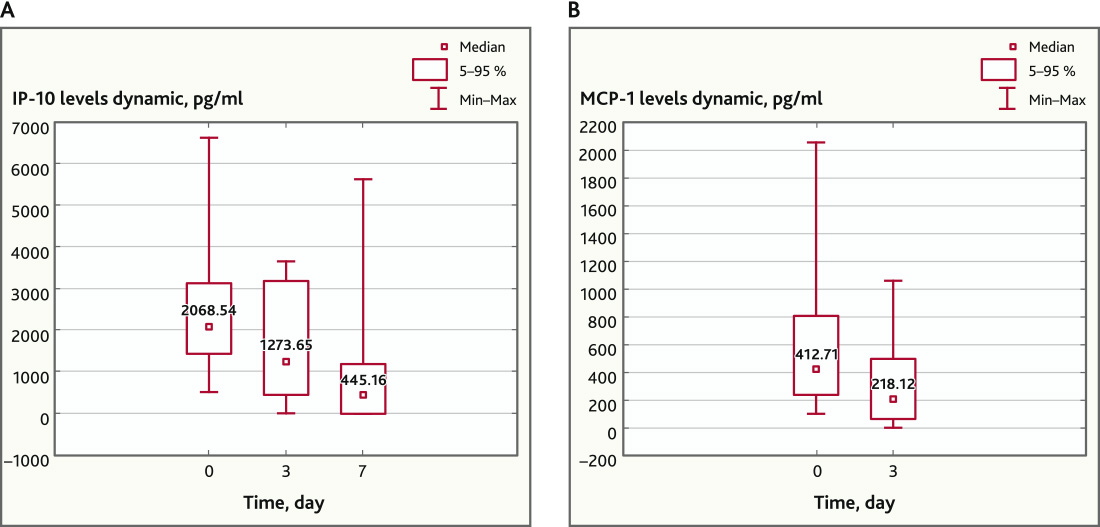
Fig. 4. IP-10 (n = 16) and MCP-1 (n = 20) levels before and after MSC application. A — IP-10 levels dynamic, pg/ml; B — MCP-1 levels dynamic, pg/ml
We analyzed the immune status of patients with COVID-19 before cell therapy and on day 7 after the MSC application in order to assess the immunomodulatory effect of MSC in ARDS. The immune status of patients was assessed by the following parameters: CD3+ (T-lymphocytes); CD19+ (B-lymphocytes); CD3+ CD4+ (T-helpers); CD3+ CD4+ CD28+; CD3+CD4+CD28+/CD3+CD4+; CD3+ CD8+ cytotoxic T cells; CD3+ CD8+ CD28+; CD3+CD8+CD28+/CD3+CD8+; CD3+ CD4+ CD25brightCD127-regulatory T cells; CD3-CD16+56+ (NK cells); CD3+ CD16+56+ T-lymphocytes with NK cell phenotype; CD3+ HLA-DR+ activated T-lymphocytes; CD3+ CD25+ activated T-lymphocytes by IL-2R; CD3+ CD38+ activated T-lymphocytes; CD3+CD4+/CD3+CD8+; CD3+CD38+/CD3+.
According to the blood test results there was a decrease in absolute count and percentage of lymphocytes in patients of both groups at the time of inclusion. Thus, in the main group, this indicator initially was 0.763 (0.294; 1.008) × 109/l. On the day 7 after the MSC application there was an upward trend in the absolute count of peripheral blood lymphocytes [1.421 (0.911; 1.869) × 109/l], however, this indicator remained below normal. Data from numerous studies evaluating the immunomodulatory effect of MSC in ARDS associated with COVID-19 indicate that CD3+ lymphocytes and their subpopulations are the most informative immunological markers [19–21]. In patients with COVID-19 in the main group, before the MSC application, we observed a statistically significant decrease in both the total number of lymphocytes in peripheral blood and their individual subpopulations, compared to normal values (p < 0.05, n = 18, Mann—Whitney U-test) (Table 3).
Table 3. Specification of T-lymphocyte subpopulations in COVID-19 patients in comparison with normal
| Lymphocyte subpopulations | Norm/Rate (minimal rate), ×109/l | Value in patients with COVID-19, ×109/l | Significance level (p-value) |
| CD3+ | 1.1 | 0.47 | 0.0001 |
| CD3+ CD4+ | 0.7 | 0.3 | 0.0008 |
| CD3+ CD8+ | 0.5 | 0.15 | 0.0008 |
| CD3+ СD25+ activated by IL-2R | 0.1 | 0.09 | 0.009 |
| CD3 + СD38+ activated | 0.5 | 0.26 | 0.0005 |
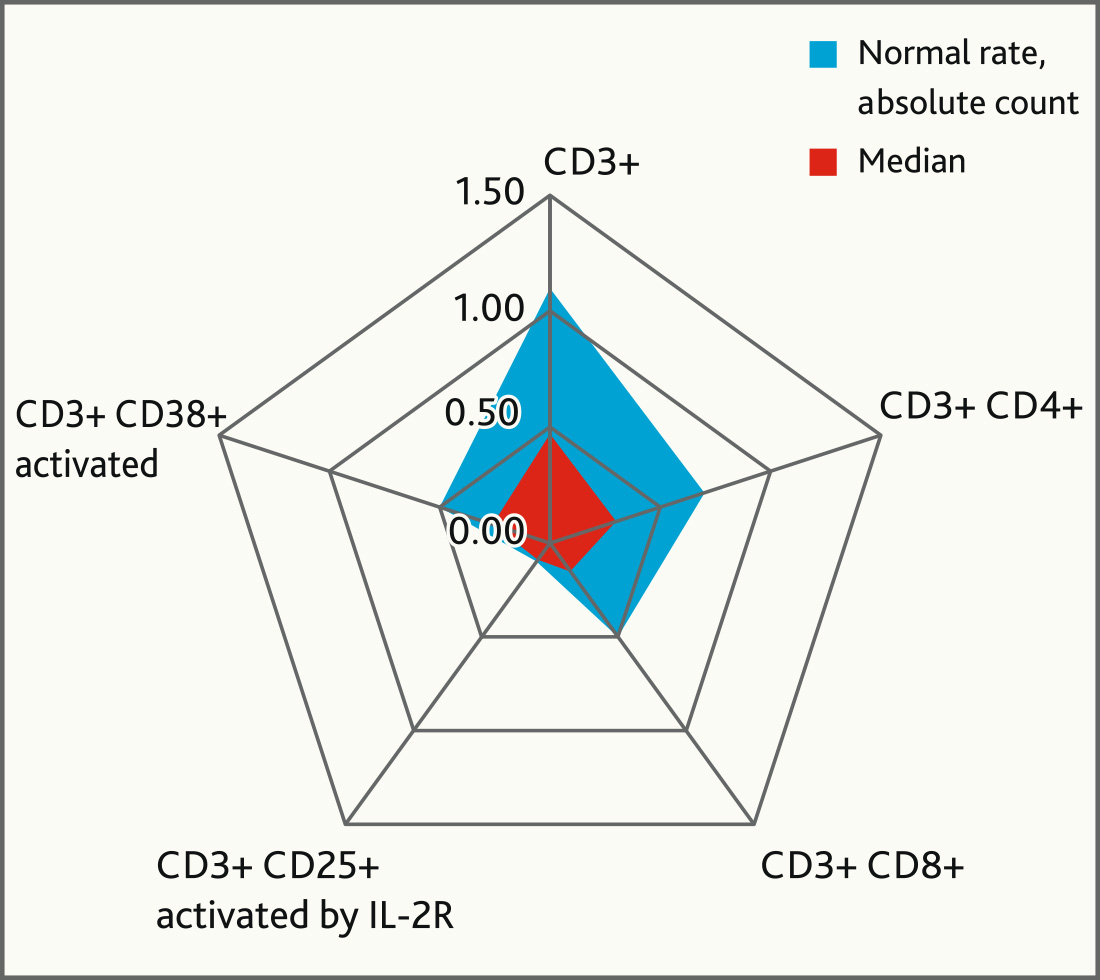
Fig. 5. Immunological marker levels before MCS application in comparison with normal levels
Comparative analysis of the immune status indicators in patients of the main group before and after MSC application did not show statistically significant differences in the studied parameters (p > 0.05, n = 18, Wilcoxon Matched Pairs Test) (Table 4). Lack of dynamics on day 7 after MSC application, apparently, is due to the insufficient observation period. All patients had severe lymphopenia at the time of inclusion in the study. It is possible that in order to obtain statistically significant differences, a longer observation period after MSC application is required.
Table 4. Comparative specification of T-lymphocyte subpopulation in main group before and after MSC application
| Lymphocyte subpopulations | Before MSC application, ×109/l | After MSC application, ×109/l | Significance level (p-value) |
|---|---|---|---|
| CD3+ | 0.48 | 0.58 | 0.45 |
| CD3+ CD4+ | 0.3 | 0.31 | 0.47 |
| CD3+ CD8+ | 0.15 | 0.19 | 0.33 |
| CD3+ СD25+ activated by IL-2R | 0.09 | 0.13 | 0.77 |
| CD3 + СD38+ activated | 0.26 | 0.21 | 0.95 |
The dynamic of clinical data in the groups was comparable, however, there were remarkably differences in the rate of Oxygenation index from day 0 — 117.5 (117.5; 182.0) to day 3 — 159.0 (110.0; 200.0) (p = 0.159, n = 23, Wilcoxon Matched Pairs Test), day 5 — 198.0 (137.5; 250.0) (p = 0.003, n = 23, Wilcoxon Matched Pairs Test) and day 7 — 217, 0 (150.5; 280.0) (p = 0.001, n = 23, Wilcoxon Matched Pairs Test) in the main group.
There was no significant increase in the oxygenation index dynamics in the control group: day 0 — 130 (78.0; 153.0), day 3 — 129.0 (101.0; 190.0) (p = 0.139, n = 16, Wilcoxon Matched Pairs Test), day 5 — 126 (97.0; 214.0) (p = 0.091, n = 16, Wilcoxon Matched Pairs Test), day 7 — 142 (99.0; 235.0) (p = 0.062, n = 16, Wilcoxon Matched Pairs Test) (Fig. 6).
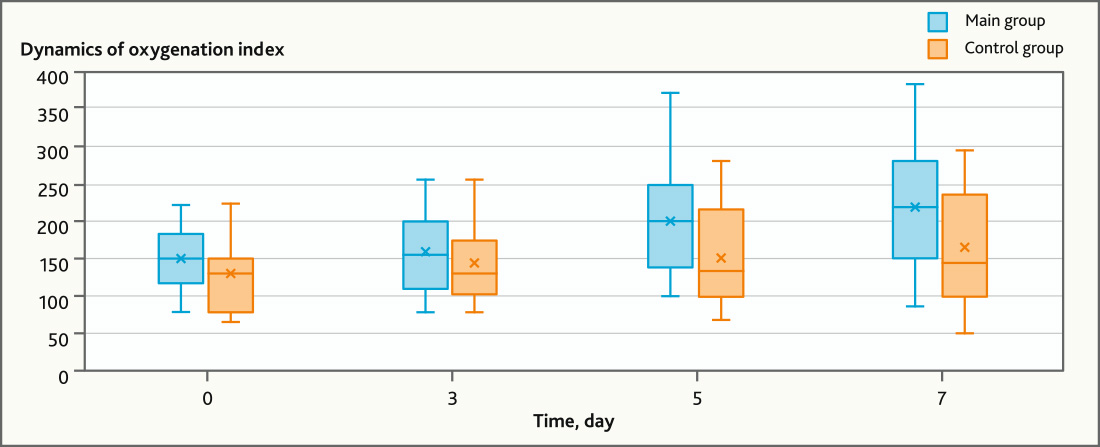
Fig. 6. Dynamics of Oxygenation index in main and control groups of patients with ARDS
Admission time in the DAIC No. 2 was comparable for both groups. However, while analyzing the use of different kinds of respiratory support we found that the number of days without mechanical ventilation was statistically lower in patient of the main group who received MSC therapy. Patients survival rate in the main group was also 16% higher than in control group (60% and 44% respectively), however the established differences were not statistically significant (р > 0.05) (Table 5).
Table 5. Outcomes in main and control group
| Main group, n = 23 | Control group, n = 16 | p-value | |
|---|---|---|---|
| Survival rate, n (%) | 14 (60%) | 7 (44%) | 0.339 |
| Days without mechanical ventilation, number of days | 9 (5; 11) | 5.5 (2.75; 10.25) | 0.037 |
| Admission time in DAIC No. 2, number of days | 22.7 | 23.2 | 0.360 |
A “cytokine storm” plays a central role in the pathogenesis of ARDS associated with COVID-19, accompanied by the production of various pro-inflammatory factors, which are the cause of tissue and organ damage. Due to immunomodulatory, paracrine, and regenerative capacity, MSC are able to indirectly restore the balance between pro-inflammatory and anti-inflammatory cytokines [15].
The development of a systemic inflammation and, as a result, an increase in pro-inflammatory biochemical blood markers is typical for a severe course of COVID-19 [23]. The positive dynamics of CRP level in patients with severe coronavirus infection after MSC application in our study also indicates a decrease in the inflammation signs and may be associated with the MSC’s anti-inflammatory effect. We received data on decreasing plasma levels of IL-6, IL-10, TNFα, IP-10 and MCP-1 in the patients with COVID-19 associated ARDS, on days 3 and 7 after cell therapy, and it confirms that allogeneic MSC application in patients with severe COVID-19 reduce the production of pro-inflammatory cytokines and prevent tissue damage. These results largely coincide with the other available clinical data [3, 22]. Moreover, R. Waterman et al. (2010) showed that TLR (Toll-like receptor) activation by pathogen components, such as viral RNA, increases the anti-inflammatory effect of MSC [6].
In our study we found that the statistically significant decrease in the LDH level against the background after MSC application in the main group on day 5 may indicate a decrease in the intensity of lung damage affected by inflammation and induction of tissue regeneration. Similar results were obtained in the study of F. Sánchez-Guijo et al. (2020) [14]. In general, these data fit into the current paradigm of ideas about MSC as an “ideal” contributor to the tissue repair process due to the activation of cell proliferation and directed differentiation [26–31].
The occurrence of the MSC anti-inflammatory effect in patients with severe coronavirus infection affects the ARDS course, which is confirmed by a statistically significant increase in the oxygenation index on days 5–7 in the main group of patients compared to the control group. Regarding the possible impact of MSC therapy on the outcomes of treatment of COVID-19 associated ARDS, we showed statistically significant decrease in admission time in MSC group patients, spent without mechanical ventilation. As mentioned above, survival rate in the MSC group was 16% higher than in the control group, but these differences were not significant. Besides, there were no statistically significant differences in admission time spent in the intensive care unit in both groups. It can be explained by the numerous causes that affect the severity of COVID-19 infection, which makes it difficult to interpret clinical data to determine the contribution of MSC therapy to treatment outcomes and length of admission time in the intensive care unit. Low sample group of patients is another limitation of our study.
Until now, the MSC application in the complex therapy of severe forms of coronavirus infection has been a clinical trial. The time of initiation of MSC therapy in patients with COVID-19 associated ARDS, the course duration and injection frequency, laboratory markers and comprehensive performance criteria of cell therapy, including the observation time, also need to be clarified. Thus, further research is needed in order to determine the place of MSC application in the intensive care of severe lung injury.
However, our results collectively showed that MSC application improved the outcome of COVID-19 patients by maintaining immune homeostasis, reducing inflammation, and improving lung function, which may indirectly indicate the regeneration of lung tissue.
The use of biomedical cellular product based on mesenchymal stem cells is a promising tool to correct the upregulated immune response in COVID-19 associated ARDS by reducing the level of pro-inflammatory Th1-polarized cytokines.
MSC application in the complex therapy of patients in the intensive care unit reduces the length of admission time on mechanical ventilation.
Disclosure. The authors declare that they have no competing interests.
Author contribution. All authors according to the ICMJE criteria participated in the development of the concept of the article, obtaining and analyzing factual data, writing and editing the text of the article, checking and approving the text of the article.
Ethics approval. This study was approved by the local Ethical Committee of Minsk Scientific and Practical Center for Surgery, Transplantology and Hematology Minsk, Belarus (reference number: 5-12.06.2020)
Funding source. This study was not supported by any external sources of funding.