Hematological malignancies in children are highly aggressive and may result in life-threatening conditions during clinical workup and chemotherapy cycles. Of particular concern is the fact that 40 % of children with tumors must be admitted to an intensive care unit at least once in the course of the disease, and the mortality rate in this group of patients is 27.8 % [1].
The high proliferative potential of lymphomas and the specific course of the disease significantly affect the confirmation of the diagnosis; therefore, the disease in pediatric patients is detected only when disseminated (stage III–IV). The frequency of hyperleukocytosis > 100 × 109/L in leukemia exceeds 13.5 % [2]. It is noteworthy that advanced disease and hyperleukocytosis are indicative of a significant tumor burden in children with lymphohematopoietic cancers.
Acute tumor lysis syndrome (ATLS) may occur soon after initiation of treatment for this condition. Understanding the likelihood of complicated disease enables effective prevention and treatment with minimal risks. In general, the interdisciplinary interaction of a pediatric (hematologist-)oncologist and an anesthesiologist/intensivist appears to be the key to success in resolving complex clinical issues [3, 4].
Objectives. The objective of this study was to summarize the current scientific evidence for the diagnosis and treatment of ATLS in children. The current scope of relevant publications in the aggregator of the PubMed medical database is not extensive: over the past 10 years, there have been 12 articles (the database contains 94 articles related to this clinical issue in pediatric practice). This makes the task of systematizing previously accumulated and recently obtained information even more relevant for the practitioner.
The current scientific evidence for the diagnosis and treatment of ATLS in children with hematological malignancies was analyzed following a search in the PubMed, ResearchGate, Web of Science Core Collection, and Google Scholar systems for the period from 2000 to August 2022 using the keywords “tumor lysis syndrome”, “acute tumor lysis syndrome”, “malignant lymphoproliferative diseases in children”, and “malignant neoplasms in children”. The categorical restrictions were randomized clinical trials and reviews in groups of children from birth to 18 years of age.
Review inclusion criteria were design (clinical trials described in any published international journal without language or national restrictions) and subjects (children with ATLS).
The investigators extracted the following data from the selected articles: last name and first name of the first author, name of the journal, country, year of publication, study design, results, and conclusions of the study.
The search identified 94 articles, including one systematic review with a meta-analysis and 16 eligible review articles.
ATLS can occur spontaneously or soon after the start of cytostatic induction (including hormonal) therapy in patients with acute leukemia, non-Hodgkin’s lymphomas (advanced disease), or, significantly less often, solid tumors (with a large tumor mass). Spontaneous tumor lysis has also been described during laparoscopic biopsy of an abdominal mass in patients with Burkitt’s lymphoma when intra-abdominal pressure increased during the procedure [5, 6]. In clinical practice, ATLS typically occurs after the start of anticancer treatment, when tumor cells break down rapidly due to the high sensitivity of the neoplasm to cytostatic drugs.
A number of authors, such as C. McDonnell et al., I.A. Malik et al., W.A. Osthaus et al. and E. Vassban et al., published case reports of ATLS resulting from the use of dexamethasone, with some of the cases being fatal [7–10]. Although dexamethasone-induced ATLS appears to be rare, this drug should be used with great care and caution in patients with confirmed or suspected hematopoietic malignancies.
In addition, other types of specific anticancer therapy can trigger ATLS. In particular, A.Y. Rostom et al. (2000) and N. Schiffer et al. (1999) note that this condition may occur in patients undergoing radiation therapy [11, 12]. Tumor lysis can be caused by vascular embolization [13] or treatment with radiofrequency ablation [14, 15] or monoclonal antibodies [16]. An article by M.F. Fer et al. (1984) and a literature review by M.P. Castro et al. (1999) traced an association between the use of interferons and the occurrence of ATLS; according to G.L. Deliliers et al. (2005), a life-threatening complication may result from high-dose chemotherapy with peripheral blood stem cell transplantation [17–19].
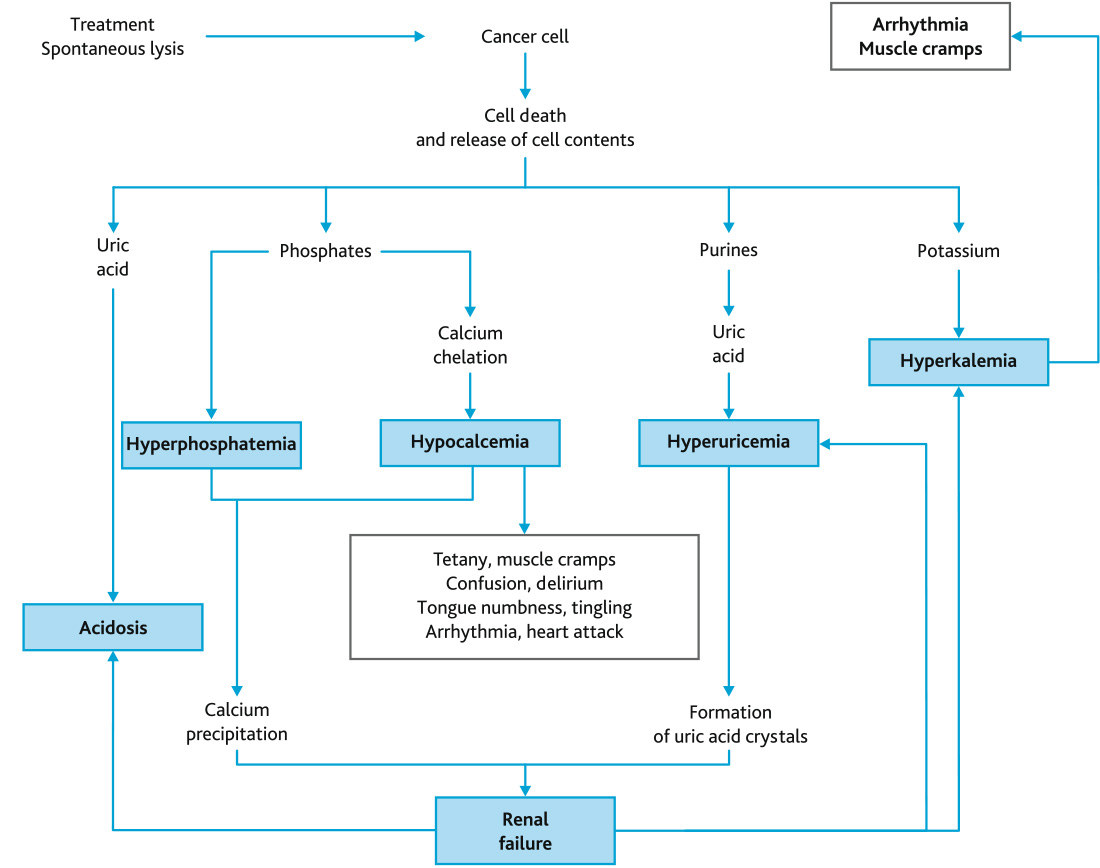
In general, laboratory signs of ATLS are observed in 4.4–53.6 % of children with leukemia or lymphomas, while severe clinical manifestations occur in 15.9 %. According to Wing Lum Cheung et al. (2014), the mortality rate is as high as 21.4 % [20]. Severe metabolic disorders result from the destruction of the cell membrane in tumor cells and the entry of intracellular electrolytes (potassium, phosphate) and metabolic products (hypoxanthine, xanthine, uric acid, lactic acid) into the bloodstream at a rate significantly exceeding their plasma clearance. The pathological changes involve spontaneous and/or treatment-induced lysis of tumor cells in children with hematological malignancies (Fig. 1).

Fig. 1. Pathogenetic mechanisms of ATLS and its clinical manifestations
The symptoms of ATLS are variable and depend on the severity of hyperkalemia and hyperphosphatemia, the concentration of tumor breakdown products, and the degree of organ dysfunction.
Active hydration and correction of blood chemistry abnormalities are the basis of ATLS treatment. Glucose 5 % and sodium chloride 0.9 % solutions (without K+, phosphate, or Ca2+) are used for these purposes. It is important that the solutions contain no potassium ions. The total volume that must be infused to increase intravascular volume and glomerular filtration is 3000–5000 mL/m2.
Urine output should be monitored in patients receiving massive infusions (every 2–4 hours); the basic output rate should be ≥ 100 mL/m2/h. If more than 400 mL/m2 is retained, furosemide must be administered within 4 hours. Loop diuretics are useful in maintaining urine output (furosemide 10 mg/kg/day), but this method is contraindicated in hypovolemia or obstructive uropathy.
As the tumor breaks down, serum uric acid increases: the acid crystallizes in the renal tubules, causing acute obstructive nephropathy and renal dysfunction. Hyperuricemia is a blood uric acid level above 476 µmol/L. Active intravenous hydration helps improve renal excretion. Patients also show elevated concentrations of xanthine and hypoxanthine, whose solubility in an alkaline medium is increased. To achieve this solubility, the infusion fluids to be administered are alkalinized with sodium bicarbonate 100–200 mmol/m2/day. However, alkaline urine promotes the formation of insoluble calcium phosphate in the tubules. Therefore, according to I. Tazi et al. (2011) and E. Rampello et al., sodium bicarbonate should be administered after the start of chemotherapy only in the event of uncontrolled acidosis [23, 24].
Allopurinol inhibits the enzyme xanthine oxidase and prevents the conversion of xanthine to uric acid. Therefore, the drug is used before the start of cytostatic therapy (if possible, 1–2 days in advance). Allopurinol should be continued after chemotherapy until the uric acid level is normalized and/or the tumor mass is significantly reduced. The dosing regimen is 300–400 mg/m2/day or 10 mg/kg every 8 hours (maximum 800 mg/day). In children weighing less than 10 kg, the dose of allopurinol is 3.3 mg/kg every 8 hours.
Uric acid levels exceeding 500 µmol/L warrant the use of rasburicase, which catalyzes the enzymatic oxidation of uric acid into an inactive and soluble metabolite, allantoin [25–28]. Rasburicase is administered at a dose of 0.2 mg/kg in combination with 0.9 % sodium chloride, 50 mL as a 30-min intravenous infusion. If elevated serum uric acid concentrations persist, another dose of rasburicase may be administered. After that, the uric acid concentration decreases quite quickly and therefore should be measured 4 hours later. Despite the high efficacy of rasburicase, rare complications (frequency less than 1 %) have been described, including anaphylactic reactions and hemolytic anemia, which should be taken into consideration in a pediatric patient’s management plan [29–31].
Thus, study results of the past 10 years question the previously recommended limited use of rasburicase (INN) even in adult patients at high risk of ATLS [32] and clearly show the benefits of allopurinol as a preventive drug for low-risk cases and urate oxidase in the treatment of patients at moderate to high risk [33].
The pathogenetic processes in ATLS patients with high potassium levels are no less severe. Conservative treatment of hyperkalemia is aimed at maintaining high urine output, ensuring hydration and eliminating acidosis. If the serum potassium level exceeds 6.0 mmol/L, insulin 0.1 U/kg is administered by intravenous infusion with 25 % glucose at a dose of 2 mL/kg or sodium bicarbonate 1–2 mEq/kg is administered intravenously. If arrhythmia is confirmed, 10 % calcium gluconate at a dose of 100–200 mg/kg IV is used.
Hypocalcemia (secondary to hyperphosphatemia) should be treated very carefully and only when symptomatic. This approach is necessary due to the high risk of formation of insoluble calcium phosphate and soft tissue calcification [20]. Active hydration is required in secondary hyperphosphatemia (blood phosphate exceeding 2.1 mmol/L). Oral aluminum hydroxide 50–150 mg/kg every 6 hours is also indicated. Hemodialysis or venovenous hemodiafiltration (HDF) is used in acute hyperphosphatemia. Hypomagnesemia is treated with magnesium sulfate solution at 0.2–0.8 mmol/kg/day.
Effective and relatively rapid treatment of metabolic disorders is possible with renal replacement therapy (hemodialysis/HDF), which quickly eliminates phosphates and uric acid [34, 35]. Absolute indications for hemodialysis/HDF include anuria or hyperuricemia, hyperkalemia (serum potassium above 6.5 mmol/L), hyperphosphatemia, or severe renal failure not responding to conservative treatment. AKI occurring due to urate nephropathy is reversible.
Plasma biochemistry markers of rapid cytolysis (potassium, phosphate, calcium, uric acid, creatinine, lactate dehydrogenase) in patients at high risk of ATLS should be tested every 4–6 hours for at least 2 days after the start of cytostatic therapy.
The development of severe acute respiratory failure indicates a condition in which all compensatory systems working at full capacity are not sufficient for adequate oxygen saturation and elimination of carbon dioxide. The clinical signs of this condition are dyspnea, cyanosis, increased heart rate, involvement of additional muscles in breathing (retraction of the intercostal, supraclavicular and subclavian spaces), moderately decreased blood pressure, and a change in the frequency and depth of respiratory movements (at a partial pressure of oxygen (PaO2) of less than 60 mmHg and/or a partial pressure of carbon dioxide (PaCO2) of more than 45 mmHg). Non-invasive or invasive ventilation may be required, depending on the degree and severity of respiratory failure.
Along with elevated potassium, phosphate, and uric acid concentrations, ATLS is associated with an increase in serum lactate [36]. Lactic acidosis has two etiological categories: type A and type B.
The main pathological events in ATLS and ways to correct them are presented in the Table 1.
Table 1. Therapeutic tactics for the main clinical, laboratory and instrumental manifestations of ATLS
| Abnormality | Monitoring and treatment principles |
|---|---|
| Breakdown of tumor cells | Infusion therapy (without K+, phosphate or Ca2+) 3 L/m2/day or 200 mL/kg/day in children weighing < 10 kg |
| Maintain urine output >100 mL/m2/h (or > 3 mL/kg/h in children weighing < 10 kg) and urine specific gravity < 1.010 g/mL | |
| Stimulation of urine output with furosemide 0.5–1 mg/kg IV. It should be remembered that furosemide is contraindicated in patients with hypovolemia or obstructive uropathy | |
| Electrolyte disorders | Uric acid, phosphate, potassium, urea, creatinine, lactate dehydrogenase measurements every 4 to 6 hours |
| Hyperuricemia > 476 µmol/L or 8 mg/dL or an increase by 25 % or more from baseline | Allopurinol 50–100 mg/m2 every 8 hours, no more than 300 mg/m2/day, or 10 mg/kg/day divided into single doses every 8 hours, maximum dose 800 mg/day |
| Rasburicase: 0.2 mg/kg 1–2 times/day IV | |
| Hyperkalemia > 6.0 mmol/L or an increase by 25 % or more from baseline | Asymptomatic: Sodium polystyrene sulfonate (1 g/kg with 50 % sorbitol) or calcium polystyrene sulfonate 15–30 g in 2–3 divided doses |
| Symptomatic: insulin 0.1 U/kg IV and 25 % dextrose 2 mL/kg or sodium bicarbonate 1 to 2 U/kg IV | |
| Arrhythmia: calcium gluconate 100–200 mg/kg by slow intravenous infusion (in a separate lumen of the central venous catheter; do not mix with sodium bicarbonate) | |
| Hyperphosphatemia > 2.1 mmol/L or an increase by 25 % or more from baseline | Phosphate binders: Aluminum hydroxide 50–150 mg/kg/day orally |
| Hemodialysis/hemodiafiltration in seriously ill patients with severe electrolyte disorders | |
| Hypocalcemia < 1.75 mmol/L or a decrease by 25 % or more from baseline | Symptomatic: calcium gluconate 50–100 mg/kg IV |
| Renal replacement therapy (hemodialysis/hemodiafiltration) | Indications:
|
An understanding of the mechanisms and risk factors of ATLS and timely clinical, laboratory, and instrumental monitoring of vital signs result in timely detection of this life-threatening complication, and a state-of-the-art armamentarium of drug therapies and extracorporeal methods enables effective treatment.
The development of ATLS in some patients treated for leukemia or lymphoma is still among the most relevant issues in a pediatric specialist’s practice. Conservative prevention of ATLS (infusion therapy, allopurinol and rasburicase, cytoreductive prephase of anticancer treatment) is effective in 93.4–93.6 % of patients [37]. However, in 8.8–21.4 % of patients, ATLS develops rapidly, precluding the use of conservative options for the control of potassium, phosphate and uric acid levels and the treatment of AKI [20]. Such a wide statistical range of treatment resistance can be explained by the lack of standard diagnostic criteria for ATLS, differences in prevention protocols and patient cohorts, different ages and disease stages of patients, delays in diagnosis and initiation of chemotherapy, and the absence of effective preventive therapy (e.g., rasburicase) in some countries. In turn, the required intensive care and anticancer treatment promote infectious complications and multiple organ failure, which increase the probability of a fatal outcome.
Further study of the pathogenetic mechanisms of ATLS and the development of drugs affecting the key stages of the elimination of tumor cell breakdown products, along with multidisciplinary management of critically ill patients with hematological malignancies, will increase the effectiveness of treatment and improve survival.
Disclosure. The authors declare that they have no competing interests.
Author contribution. All authors, according to the ICMJE criteria, participated in developing the concept of the article, obtaining and analyzing factual data, writing and editing the text of the article, and checking and approving the text of the article.
Funding source. This study was not supported by any external sources of funding.